ANTI CANCER
DRUGS
1
INTRODUCTION
Definition: Cancer is a disease characterized by uncontrolled multiplication
and spread of abnormal forms of the body's own cells.
The terms cancer, malignant neoplasm (neoplasm simply means 'new
growth') and malignant tumor are synonymous.
TWO TYPES OF TUMOUR
1 BENIGN TUMOUR
2 MALIGNANT TUMOUR
2
THE PATHOGENESIS OF CANCER.
Cancer cells manifest four characteristics that distinguish them from normal
cells
1.uncontrolled proliferation
2.dedifferentiation and loss of function
3.invasiveness
4.Metastasis
3
General principles for cancer therapy
• The cells of a solid tumour can be divided into 3
compartments:
• Continuously dividing cells
Compartment
A
Compartment
B
• Resting cells in G0 phase
• Have an ability to enter the cell cycle
• No ability to divide
Compartment
C
4
• Only the cells in the compartment A i.e. the dividing cells are
susceptible to the main currently available cytotoxic drugs. But
these cells are only 5% of the entire tumour.
• The cells in the compartment B tend to pose a problem as
they are not susceptible to cancer chemotherapy but are likely
to enter the compartment A following the course of
chemotherapy.
• Most of the currently available anti cancer drugs are
antiproliferative. They have no specific inhibitory effect on
invasiveness, the loss of differentiation or the tendency to
metastatise. Their main action is on S phase of the cell cycle
and hence results into the DNA damage causing apoptosis.
5
• Lastly , the cancer chemotherapy also acts on the normal tissues
and thereby they produce a some general toxic effects like :
• bone marrow suppression (myelosuppression) resulting in decreased
leucocyte production and hence increased resistance to infection.
• Impaired wound healing
• Loss of hair
• Damage to the gastrointestinal epithelium
• Growth retardation in children
• Sterility
• Teratogenecity
• They can themselves be carcinogenic
• Kidney damage due to excess urate deposition in the tubules ( due to
extensive purine catabolism)
• Severe nausea and vomitting ( reduced patient compliance)
6
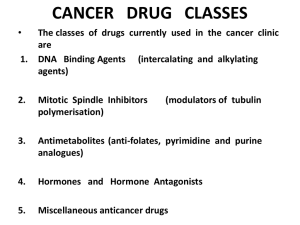
DRUGS USED IN CANCER
CHEMOTHERAPY
1. Cytotoxic Drugs
•
•
•
•
•
Alkylating agents
Antimetabolites
Antimitotics
Cytotoxic antibiotics
Plant derivatives
2. Hormones
3. Miscellaneous agents
7
Alkylating agents
• The nitrogen at position 7 (N7) of guanine is strongly
nucleophilic and is the main target for the alkylating
agents. N1 and N3 of adenine and N3 of cytosine
may also be affected.
• Most of these agents are bifunctional i.e. they have
two alkylating groups and can cause inter as well as
intrachain cross-linking.
• This can not only interfere with transcription but
also replication .
8
• Other effects of alkylation at guanine N7 are excision of
the guanine base with main chain scission, or pairing of the
alkylated guanine with thiamine instead of cytosine and
eventually substitution of the GC pair by an AT pair.
• The effects of this alkylation are manifested during S
phase, resulting in block at G2 phase and subsequent
apoptotic cell death.
• Side effects:
• bone marrow depression,
• git disturbances at very high doses.
• neurotoxicity
• With prolonged use it can lead to sterility
9
10
Intrastrand and interstrand crosslinking in DNA
Types of alkylating agents are:
1. Nitrogen Mustards
Cyclophosphamide
Ifosphamide
Chlorambucil
Melphalan
Mecholrethamine
Bendamustine
Glufosfamide
3. Agents with trizene group
Dacarbazine
Procarbazine
Timozolamide
2. Nitrosoureas
Lomustine
Carmustine
Semustine
Streptozocine
5. Agents with sulfate group
Busulfan
4. Ethelenamines
Thiotepa
Hexamethylmelamine
Platinum compounds:
Cisplatin, carboplatin
11
Cyclophosphamide/ifosfamide are prodrugs
Hepatic cytochrome oxidase
Metabolized to aldophosphamide
Tissues
Acrolein
causes hemorrhagic cystitis
Treatment: Intake of fluids and
–SH group donors like Mesna
Phosphoramide mustard
Alkyl group which causes
N7 guanine and causes
inhibition of
DNA
12
Other imp points of alkylating agents:
Streptozocine:
• destroys the beta cells of pancreas so approved for treatment of
insulinoma. Also used to induce diabetes mellitus in experimental
animals.
Busulfan:
• Selective action on bone marrow, depressing the formation of
granulocytes and platelets in low dose and red blood cells in high
dose.
• Use: chronic granulocytic leukemia, as a preparative for bone
marrow transplantation
• Main side effect is pulmonary fibrosis and neurotoxicity (seizures)
• Also can cause adrenal suppression
Estramustine: Estrogen + mechlorethamine
• Approved for the treatment of prostatic carcinoma
• S/E – Gynaecomastia, loss of libido, thromboembolism
13
Cisplatin:
• M/A:
• it has a platinum atom surrounded by two chloride atoms and two ammonia
groups. When it enters the cell the cl- group dissociates leaving the reactive
complex to interact with DNA. It causes intrastrand crosslinking between N7
and O6 of adjacent guanine molecules leading to denaturation of DNA.
• Side effects:
• nephrotoxicity,(hydration and diuresis required)
• Severe nausea and vomiting (ondansetron given)
• Tinnitus and hearing loss
• Low myelotoxicity
• Others: peripheral neuropathies, huperuricaemia, anaphylactic
reactions.
• Use: solid tumours of testes and ovary
Carboplatin: derivative of cisplatin
• More myelotoxicity
• Less neurotoxocity, nephrotoxicity , ototoxicity, nausea and vomiting.
• Cisplatin – max S/E and min potency
• Carboplatin – intermediate
• Oxaliplatin – min S/E and max potency
14
Antimetabolites
• These drugs act in the s-phase of the cell cycle so only dividing
cells are responsive
Folic acid analogues Purine analogues Pyrimidine analogues
Methotrexate
6-mercaptopurine 5-flourouracil
Pemetrexed
6-thioguanine
Capecitabine
cladrabine
Gemcitabine
Pentostatin
Cytarabine
Fludrabine
Methotrexate:
• Inhibitor of dihydrofolate reductase enzyme which is required
for synthesis of thymidylate and hence DNA formation.
• At high dose – by diffusion enters the normal cells and inhibits
DNA formation in the normal cells
15
S/E: BM suppression, diarrhoea, mucositis due to inhibition of rapidly
proliferating cells
Drug of choice for overdose:
• Folinic acid or leucovorin or citovorum factor. It directly gets
converted to tetrahydrofolate bypassing the requirement of
dihydrofolate reductase.
• On the surface of cancer cells, there is an influx pump which takes in
methotrexate at low dose. Inside the cells it binds with
polyglutamine
Forms methotrexate polyglutamine complex which serves as
reservoir of methotrexate leading to long lasting effect.
• MTX has affinity for cells having high chromosome no. like placental,
fetal and germ cells so it is drug of choice for choriocarcinoma in
which single drug treatment is used.
16
• Eliminated by kidney- tubular secretion by acidic pumps.
• NSAIDS can saturate these pumps leading to decreased secretion of
MTX causing toxicity.
Uses:
• Almost all types of cancers, Burkitt’s lymphoma, choriocarcinoma,
breast carcinoma, osteosarcoma.
• It also has immunosuppressive property so it is used for organ
transplant, RA, IBD, Psoriasis, Ectopic pregnancy.
Resistance: - mutation of influx pumps
- induction of efflux pumps
- mutation of DHFRase enzyme
Pemetrexed:
• inhibitor of thymidine synthase.
• Folic acid or vit B12 can reduce the toxicity of pemetrexed
• Approved for tumour of mesothelioma
• S/E- peripheral neuropathy in hand and foot region called as handfoot syndrome.
17
Mechanism of action methotrexate and fluorouracil
18
Pyrimidine analogues:
• 5-FU is the prodrug
• Active metabolite is 5- fluorodeoxyuridine monophosphate
• Cells are unable to differentiate between 5F-dUMP and dUMP
5F-dUMP
CH3
thymidine synthase
Fluorothymidine
Inhibits DNA synthesis because of incorporation of modified
neucleotide
• Leucovorin given with 5FU increases formation of
fluorothymidine causing increased effect of 5FU. This concept
is used in colorectal cancer.
FOLFOX
regimen of colorectal cancer. FOL- Folinic acid
FOLFIRI
F- 5FU, OX- oxaliplatin, IRI- irinotecan
19
• Metabolized at the level of lung tissue by endothelial
enzymes.
• Given by i.v route
• Alternative: capecitabine which is prodrug of 5FU given by oral
route.
Gemcitabine/Cytarabine:
• Act as prodrugs. Combines with 3PO4 groups and converts to
Gemcitabine triphosphate
Cytarabine triphosphate
modified nucleotides which with
the help of DNA polymerase gets
incorporated into DNA inhibiting
DNA synthesis
Gemcitabine use: non beta cells cancer of pancreas
S/E: flu like symptoms
20
Purine analogue:
6-MP ( prodrug)
6-methylmercaptopurine
HGPRTase
Purine structure
DNA polymerase
Incorporation in DNA. So inhibition of DNA synthesis.
• Metabolized by xanthine oxidase to form uric acid.
• Some part is metabolized by methyl transferase enzyme.
6-Tiagabine:
acts in the same way as 6-MP but metabolized
only by methyl transferase enzyme.
Azathioprine: prodrug of 6-MP. Used only as
immunosuppressive.
21
Pentostatin: inhibitor of adenosine deaminase. Used in hairy
cell leukemia.
Cladribine: used in hairy cell leukemia
Antimitotics
1. Vinka alkaloids
2. Texans
3. Ixebepilone
Vinka alkaloids:
• vincristine, vinblastine, vinorelbine
• MOA: inhibition of spindle formation
• S/E: - SIADH
- Bone marrow suppression
- Peripheral neuropathy.
22
Taxanes: Paclitaxel, docitexal, cabazitexal
• MOA: inhibition of spindle formation
• Approved for the treatment of breast carcinoma
• Paclitaxel + carboplatin: treatment of choice in ovarian cancer.
• S/E: Hypersensitivity reaction and to inhibit it paclitexal is
combined with albumin to form abpaclitaxel
• bone marrow suppression
• peripheral neuropathy
Ixebepilone: MOA same as texans but more potent than
paclitexal so used in treatment of paclitexal
resistant breast cancer
Advantage – no hypersensitivity reaction
23
Anti tumour antibiotics
1.
2.
3.
4.
5.
Anthracycline antibiotics
Mitoxantrone
Mitomycin c
Bleomycin
Actinomycin D
Most imp mechanism of action – Free radicle formation
S/E:
• Cardiotoxicity – cardiomyopathy after 2-3 months.
Treatment– dexrazoxane, alpha – tocoferol – they are free
radicle scavengers
• Bone marrow suppression
24
ANTHRACYCLINES:
• Doxorubicin,Idarubicin, epirubicin, aclarubicin, mitoxantrone.
• Mechanism of action of Doxorubicin: inhibits both DNA and
RNA synthesis by an effect on topoisomerase II (DNA gyrase)
• Given by i.v. infusion
• Epirubicin is less cardiotoxic than doxorubicin.
• Mitoxantrone has dose-related cardiotoxicity .
Dactinomycin:
Mechanism : it intercalates in the minor groove of DNA and
interferes with the movement of RNA polymerase and thus
prevents transcription.
25
• Red urine
• Radiation recall phenomenon – seen after many years of
radiation. Epithelial damage and vesicles seen at the same site
after taking drugs
• Most common S/E – Nausea, vomitting, diarrhoea
MITOXANTRONE
• Same as above but cardiotoxicity is rarely seen.
• Associated with blue urine, blue nails, blue sclera
• Indication – Multiple sclerosis
26
MITOMYCIN C
• Functions as a bifunctional alkylating agent
• Very potent drug but causes toxicity when used systemically.
• So not used via systemic route.
Uses
• Tracheal stenosis and
• Oesophageal stenosis
• Superficial bladder cancer
BLEOMYCIN
• With the help of Fe2+ bleomycin converts O2 to free radicles
and causes DNA damage. It is the only anticancer drug that
can be given via any route.
27
• Metabolized by bleomycin hydrolase which is present all over
the body except skin and lung. So the main S/E is lung and skin
fibrosis.
• It causes little myelosuppression.
• RADIOACTIVE IODINE : I131: Used in treatment of thyroid
tumours.
28
• TOPOISOMERASE INHIBITORS
Etoposide
Teniposide
(Topoisomerase II)
Irinotecan
Topotecan
(TopoisomeraseI)
campothecins
• All drugs eliminated by kidney. Caution in CRF
• Except Irinotecan which is a drug metabolized by liver to
active SN38 which is eliminated by kidney.
• Cholinergic properties – diarrhoea present since day 1 of
therapy.
• USE – colorectal carcinoma.
29
Hormones:
• Glucocorticoids: They have inhibitory effect on lymphocyte
proliferation
• Used in leukemias and lymphomas
• Estrogens: Fosfeterol (a prodrug which is activated by acid
phosphatase in prostatic tissue ) block the effect of androgens
in androgen-dependent prostatic tumours.
• Estrogen can also be used to recruit resting mammary cells
from compartment B to compartment A thus allowing greater
killing efficiency of cytotoxic drugs.
• Progesterons: Megesterol and medroxyprogesterone have
been useful in endometrial neoplasms and renal tumours.
30
• Gonadotrophin- releasing hormone analogues:
• Goserelin can inhibit gonadotrophin release.
• Used to treat advanced breast cancer in postmenopausal women
and prostate cancer.
• Analogue of somatostatin:octreotide is used to treat various
hormone secreting tumours of the git such as VIPomas,
glucagonomas, gastrinomas. These tumors express
somatostatin receptors, activation of which inhibits cell
proliferation and hormone secretion.
31
Hormone antagonists:
• Anti-estrogens: Tamoxifen is effective in hormone dependent
breast cancer. In breast tissue, Tamoxifen competes with
endogenous oestrogens for oestrogen receptors and inhibits
transcription of oestrogen- responsive genes.
• Also has cardioprotective effect by preventing oxidation of
LDL.
• Anti-androgens: Flutamide and cyproterone are used in
prostate tumours.
• Adrenal hormone synthesis inhibitor: Formestane acts at a
late stage of sex hormone synthesis by inhibiting enzyme
aromatase. Trilostane and aminoglutethimide also inhibit sex
hormone synthesis.
32
Miscellaneous agents:
L-ASPERGINASE(Crisantaspase)
• Only enzyme used as anticancer drug.
• Metabolize aspergine to urea or ammonia
• Normal cells can form all aspergine on their own but cancer cells
cannot form their own aspergine and depend on plasma aspergine.
L-asperginase depletes plasma aspergine causing cancer cell death.
USE- Leukemias
S/E
• Hyperammonemia
• Protein deficiency
• Clotting and anticlotting factors are proteins so clotting disturbances
• Hupoalbuminemia
33
HYDROXYUREA
• Inhibitor of ribonucleotide reductase enzyme which converts
ribose sugar to deoxyribose sugar incorporated in DNA
• Acts on the S phase of the cell cycle
• Side-effect: myelosupression
34
Miscellaneous agents:
• Imatinib mesylate: it is a small molecule which inhibits
signalling pathway kinases. It inhibits platelet- derived growth
factor.
• It also inhibits cytoplasmic kinase which is a unique factor in
pathogenesis of chronic myeloid leukemia and hence licensed
for the treatment of this tumour.
• Biological response modifiers: agents which enhance the host
response.
• Examples: interferon γ, aldesleukin and tretinoin.
35
Monoclonal antibodies
Rituximab:
• Attaches to CD20 proteins on B cells and kills B cells by complement
mediated lysis or by inducing apoptosis.
• Use: B cell lymphoma
• Side effects: hypotension, chills and fever initially
• Hypersensitivity reaction
• May make cardiovascular diseases worse
Trastuzumab:
• Binds to a protein called Her2/neu. (member of the epidermal
growth factor receptor family- receptors with integral tyrosine
kinase activity)
• Use: in some patients with breast cancer, these receptors are
overexpressed
• Side effects : similar to rituximab
36
• GEMTUZUMAB-OZAGAMYCIN COMBINATION
• MOA – DNA inhibition by formation of free radicles.
CELL CYCLE SPECIFIC agents
NON CELL CYCLE SPECIFIC agents
G1/S –Etoposide
Topoisomerase inhibitors
S – Antimetabolite
Hydroxyurea
Alkylating agents
G2/M – Etoposide
Bleomycin
All anticancer antibiotics except
bleomycin
M – vinka alkaloids
taxens
Ixebepilone
Estramustine
Platinum compounds
37
REGIMENS FOR DIFFERENT
TUMOURS:
REGIMEN
DRUGS
COAP
Cyclophosphamide+Vincristine+
cytarabine+prednisolone
POMP
Prednisolone+Oncovin(Vincristine)+
Mtx+Purinethol(6-MP)
CART
Cytarabine+Asparginase+Rubidomycin
(daunorubicin)+ 6-TG
MOPP
Mechlorethamine+Oncovin+
Procarbazine+Prednisolone
VAMP
Vincristine+Amethopterine(MTX)+ 6MP+Prednisolone
38
MALIGNANCY
FIRST LINE
THERAPY
SECOND LINE
DRUGS
Acute leukaemias
VAMP
Cytarabine, Lasparginase, Mtx,
Doxorubicin
Hodgin’s Disease
MOPP
Bleomycine,
Procarbazine,
lomustine,
ifosphamide,
doxorubicin
Prostate carcinoma
Flutamide+GnRH
agonist
Doxorubicin,
cisplatin,
cyclophophamide,
5-FU
Breast carcinoma
Tamoxifen, Mtx,
5-FU
Prednisolone,
Vincristine,
Paclitaxel,
cyclophosphamide,
Mitoxantrone
39
Malignancy
First line drugs
Second line drugs
Ovarian carcinoma
Cisplatin,
carboplatin,
paclitaxel,
cyclophosphamide,
Doxorubicin
Melphalan,
Chlorambucil, 5-FU,
Mtx, Vincristine,
Topotecan
Endometrial cancer Progestin,
tamoxifen
Doxorubicin,
cisplatin
Testicular tumours
Mtx, bleomycin,
etoposide,
cisplatin,
carboplatin
Actinomycin,
ifosfamide,
doxorubicin,
vinblastine,
melphalan
Lung cancer
Cyclophosphamide, Carboplatin, Mtx,
Vincristine,
Lomustine,
Doxorubicin
topotecan,
etoposide
40
Dealing with side effects:
• EMESIS:
• Mainly seen with cisplatin and other alkylating agents.
• Treatment: ondansetron or granisetron can be used
• Metoclopromide can also be used . But since it causes extrapyrimidal side effects, diphenhydramine can be used instead.
41
• MYELOSUPPRESSION
• Removing some of the patients bone marrow prior to giving
cytotoxic drugs, purging it of the cancer cells and replacing it later
• Administration of Molgramostim, then harvesting stem cells from
blood and multiplying them in-vitro with the relevant
heomopoietic growth factors.
• Introducing mutated gene into the extracted bone marrow which
confers multidrug resistance so that when this marrow is
replaced the normal cells remain resistant to cytotoxic drugs but
the cancer cells don’t .(a possibility)
42
Newer anti cancer targets
• The Ras proteins- Farnesyl transferase inhibitors are in
development
• Tyrosine Kinase : Agents interfering with the epidermal growth
factor receptor tyrosine kinase activity can be developed.
• Cyclin and cyclin dependent kinases: Flavopiridol
• Telomerase inhibitors
• Angiogenesis and matrix metalloproteinase inhibitors:
• Cyclo-oygenase inhibitors: COX-2 is overexpressed in many
cancer cells. Cox-2 inhibitor celecoxib has shown to reduce
mammary tumours in animal models.
• P53 gene: cancer cells carry a mutated p53 gene
• Antisense oligonucleotides: inhibit gene expression in tumour
cells.Example : augmerosen downregulates Bcl-2
43
REFERENCES:
1. Rang HP, Dale MM, Ritter JM, Moore PK Pharmacology. 5th
edition. New Delhi: Elsevier publication; 2002, p. 693-709
2. Joel HG, Lee LE, Gilmann Ag. Goodmann and Gillmann’s The
pharmacological basis of Therapeutics. 11th edition.McGraw-Hill publication; 2006, p.670-680
3. Tripathi KD, Essentials of Medical Pharmacology. 6th edition.
Jaypee Brothers Medical Publishers(P)LTD; 2008, p.:819-836
44