17 January 2013
1
Follow-up to Previous Reviews
Immune Globulin (IV and SC)
Atopic Dermatitis
2
Immune Globulin (IV and SC)
Additional Responses to DUR letters sent in August 2012
One doctor’s office had charged Idaho Medicaid for immune
globulin (brand name Privigen) 500mg as a single dose for
four separate patients.
Patients had actually received promethazine 50mg
injectable.
Doctor’s office has been asked to correct billing error
3
Immune Globulin (IV and SC)
Additional Responses to DUR letters sent in August 2012
Adult male patient receiving immune globulin 1000mg/kg
monthly for chronic inflammatory demyelinating
polyradiculoneuropathy (CIDP). This patient is also a poorly
controlled diabetic patient who has gained more than 100
pounds over the past year, resulting in significantly higher
dosages. Standard of care is to use either ideal body weight or
adjusted body weight (defined as ideal body weight plus 50%
of the difference between actual and ideal body weight) in
obese patients. Dose for December 2012 was reduced from
128gm ($14,930) to 100gm ($11,664). In addition, early refill
request for patient convenience was denied for December
2012.
4
Immune Globulin (IV and SC)
Additional Responses to DUR letters sent in August 2012
Pregnant woman whose first child died secondary to
congenital hemochromatosis (2008). Second child survived
(2010) – mother had been treated with IVIG weekly from
Weeks 14-37. Third pregnancy (due date February 2013) –
mother is being treated with IVIG weekly starting at week 14
(September 2012). Have approved IVIG to continue
throughout this third pregnancy.
5
Immune Globulin (IV and SC)
Recommendations from October 2012 DUR meeting
1. Require prior authorization for this expensive therapy
both on the Medical side and the Pharmacy side.
2. Check for a FDA approved diagnosis and verify clinical
benefits as well as monitor periodic IgG levels (if
applicable to diagnosis, such as
hypogammaglobulinemia).
3. Initially approve for 3-6 months with additional
documentation required after that time period to
renew the authorization.
4. Implementation date of 01/01/2013
6
Immune Globulin (IV and SC) Medical Claims
Claims paid on medical side between
8/01/2011 and 7/31/2012
$288,410
116 claims
24 patients
Average cost per prescription: $2486
7
Immune Globulin (IV and SC) Medical Claims
Requiring prior authorization for applicable J-codes
effective 01/01/2013.
Notification of new prior authorization requirements
published in Medic Aide
Pharmacy Unit will be processing these prior
authorization requests.
8
Immune Globulin (IV and SC)Pharmacy Claims
Reviewing outpatient prescription claims
between 8/01/2011 and 7/31/2012
$279,527
79 claims
14 patients
Average cost per prescription: $3538
9
Immune Globulin (IV and SC)Pharmacy Claims
Requiring prior authorization effective 01/01/2013 .
Notification letter sent out to current prescribers and
pharmacies of patients receiving immune globulin
between September – November 2012(letters sent
second week of December) and to new patients
receiving immune globulin in December 2012 (letters
sent early January 2013).
10
Immune Globulin (IV and SC)
Reference:
Intravenous Immune Globulin in Autoimmune and
Inflammatory Disease. NEJM 2012;367:2015-25.
Review article in NEJM published in November 2012.
Medicare or a commercial insurer has approved
reimbursement for such therapy [autoimmune conditions],
often conditionally, requiring documentation of
contraindications to or a lack of response to conventional
therapies.
11
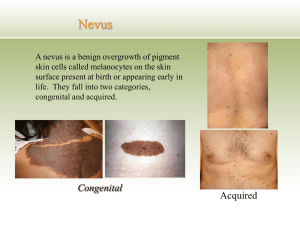
Atopic Dermatitis
The P&T Committee requested a DUR on this drug class to
include patterns of use, presence or absence of step up therapy
from steroids, specialty of prescribers and geographic region
differences of prescribing patterns. The DUR should include an
educational piece on risks of these agents compared to risks from
steroids since many practitioners seem to be using these agents
to spare patients from steroid exposure.
DUR completed April 2012 and it was felt that the medications
were being used appropriately based on the data presented and
these findings were presented to the P&T Committee.
12
Atopic Dermatitis
Treatment
Emollients are considered mainstay of maintenance therapy
Topical corticosteroids are the standard of care to which other
treatments are compared and are considered first-line treatments
for flare-ups.
Local side effects include striae, atrophy, and telangiectasia.
Systemic side effects including hypothalamic-pituitary-adrenal axis
suppression, reduced linear growth in children, and bone density
changes in adults are the most worrisome. There is no conclusive
evidence that appropriately used topical steroids cause significant
systemic adverse effects.
Topical corticosteroids should be used for the shortest duration
possible to control the flare-up.
13
Atopic Dermatitis
Treatment
Sedating antihistamines are useful when patients have sleep
disturbances and concomitant allergic conditions.
Antibiotics should be reserved for patients with acutely infected
lesions.
Topical calcineurin inhibitors should be second-line treatment for
flare-ups and maintenance.
Local side effects include skin burning and irritation. Patients should
also be counseled on proper sun protection.
Black Box Warning – discussed on next slide
14
Atopic Dermatitis
15
Atopic Dermatitis
In March 2010, the FDA issued a public health advisory about the
potential cancer risk associated with the use of Elidel
(pimecrolimus) and Protopic (tacrolimus) products applied to
the skin.
This was based off of information from animal studies, case reports
in a small amount of patients, and how the drugs work.
The FDA recommends that healthcare providers, patients, and
caregivers consider the following:
Use these products only as second-line agents as short term and
intermittent treatment.
Avoid the use in children under the age of 2.
Use for a short period of time, not continuously.
Children and adults with a weakened or compromised immune
system should not use these products.
Use the minimum amount of the products needed to control the
patient’s symptoms.
16
Atopic Dermatitis
References
Hanifin, J.M., Cooper, K.D., Ho, V.C., Kang, S., et al. Guidelines of care for atopic
dermatitis. Journal of the American Academy of Dermatology. 2004;50:391-404.
Peterson, J.D., Chan, L.S., A Comprehensive Management Guide for Atopic
Dermatitis. Dermatology Nursing. 2006;18(6):531-542.
Buys, L.M., Treatment Options for Atopic Dermatitis. Am Fam Physician. 2007;Feb
15;75(4):523-528.
Retrieved March 16, 2012.
Retrieved March 16, 2012.
Elidel [package insert]. East Hanover, NJ; Novartis Pharmaceuticals Corp.; July 2010.
Protopic [package insert]. Deerfield, IL; Astellas Pharma US, Inc.; November 2011.
17
Atopic Dermatitis
The P&T Committee asked at their October 2012
meeting for the DUR Board to look at how frequently
these medications were being filled.
A review of paid claims between 10/01/2011 and
10/01/2012 was done.
18
# of patients – both Protopic
and Elidel
Atopic Dermatitis
Paid claims between 10/01/2011 and 10/01/2012
350
300
293
250
200
150
81
100
50
23
12
7
7
0
4
3
2
1
3
# of claims
1
2
3
4
5
6
7
8
9
10
11
19
Atopic Dermatitis
Paid claims between 10/01/2011 and 10/01/2012
# of patients
250
216
200
150
100
50
0
87
50
30
16 10
3 4 2 1 2 0 3
6 2 3 3 0 2 2 1 0
Protopic
Elidel
# of claims
1
2
3
4
5
6
7
8
9
10
11
20
Atopic Dermatitis
# of claims
Age-both
Age-Protopic
Age-Elidel
1
11.58 ± 10.62
11.97 ± 12.23
11.09 ± 9.79
2
13.20 ± 15.56
19.79 ± 20.96
10.51 ± 11.77
3
10.65 ± 7.91
14.50 ± 13.70
13.06 ± 11.91
4
15.33 ± 15.09
4.00 ± 2.83
17.60 ± 15.59
5 to 11
20.48 ± 20.31
18.36 ± 19.07
19.87 ± 20.66
21
Atopic Dermatitis
Average Age of Patient
25
20
15
10
5
0
1
2
3
4
5 to 11
# of claims
Both
Protopic
Elidel
22
0
# claims for Elidel/Protopic
Patient 399
Patient 203
Patient 75
Patient 156
Patient 73
Patient 127
Patient 8
Patient 196
Patient 2
Patient 5
Patient 201
Patient 269
Patient 64
Atopic Dermatitis
Patients with more than 6 claims for Elidel/Protopic
30
25
20
15
10
5
# claims for steroid
23
Atopic Dermatitis
More than 6 claims for Elidel or Protopic in one year
Patient Number
Patient Age
(years)
Drug
# Claims for
Elidel/Protopic
# Claims for
Topical Steroid Prescriber
Diagnosis in
Electronic Profile
64
15
Elidel
11
24
Dermatologist
269
14
Elidel
11
11
P.A.
atopic dermatitis
atopic dermatitis,
eczema
201
9
Elidel
11
3
Family Medicine
No derm diagnosis
5
38
Protopic
10
0
Family Medicine
No derm diagnosis
2
65
Elidel
9
15
N.P.
196
9
Protopic
9
9
Dermatologist
eczema
atopic dermatitis,
eczema
8
61
Protopic
9
0
Family Medicine
eczema, psoriasis
127
12
Elidel
9
0
Pediatrics
No derm diagnosis
73
13
Elidel
8
8
Family Medicine
156
10
Protopic
8
8
Allergist
75
41
Protopic
8
1
Family Medicine
eczema
atopic dermatitis,
eczema
atopic dermatitis,
eczema
203
10
Elidel
7
11
Dermatologist
399
4
Elidel
7
0
Pediatrics
eczema
atopic dermatitis,
eczema
24
Atopic Dermatitis
Patients with more than 6 claims for Elidel/Protopic
Steroid Claims
7
# of patients
6
6
5
4
3
3
2
1
3
1
0
0
Pediatrics or Family
Medicine
0 - 3 claims
0
Dermatologists
P.A., N.P., Allergist
more than 3 claims
25
Atopic Dermatitis
Patients with more than 6 claims for Elidel/Protopic
# of patients
2
1
0
Dermatologist
AD
P.A.
AD & eczema
Family Medicine
N.P.
eczema & psoriasis
Pediatrics
eczema
Allergist
No Derm Dx
26
Atopic Dermatitis
Paid claims between 10/01/2011 and 10/01/2012
350
317
# of claims
300
250
200
130
150
100
71 61
50
59
67
27
15 12
0
30gm
60gm
100gm
2
2
120gm
1
300gm
Quantity Dispensed
Elidel
Protopic 0.03%
Protopic 0.1%
27
Atopic Dermatitis
Conclusions:
Overall only 13 of the 436 patients (3%) filled their
Elidel/Protopic more than once every other month.
Of those 13 patients, 7/13 were filling prescriptions for
topical steroids at least as often as prescriptions for
Elidel/Protopic.
For the 6 patients with no or infrequent topical steroid
fills over the same time period, should any action be
taken (e.g. send a DUR letter asking for chart notes)?
Should limits be placed on how often Elidel/Protopic is
dispensed?
28
Atopic Dermatitis
Recommendations of DUR Board
29
Current Interventions/Outcomes
Studies
P&T Committee Narcotic Analgesic Studies
National Summit on Opioid Safety
Psychotropic Medications in Foster Children
Two (2) or more concomitant antipsychotics
Synagis Update
Revatio
30
National Summit on Opioid Safety
October 31- November 1, 2012
Seattle, Washington
31
Principles for more selective and
cautious opioid prescribing*
Principles for All Chronic Non-Cancer Pain Patients
1.
2.
3.
4.
Self-care is the foundation for effective chronic noncancer pain care
Your relationship with the patient supports effective
self-care
Guide care by progress toward resuming activities
Prioritize long-term effectiveness over short-term pain
relief
* These principles are not intended for palliative care of chronic pain at end of life.
32
Principles for more selective and
cautious opioid prescribing*
Principles When Considering Long-term Use of Opioids
1.
2.
3.
4.
5.
6.
Put patient safety first
Think twice before prescribing long-term opioids for
axial low back pain, headache and fibromyalgia
Systematically evaluate risks
Consider intermittent opioid use
Do not sustain opioid use long-term without decisive
benefits
Keep opioid doses as low as possible
* These principles are not intended for palliative care of chronic pain at end of life.
33
Principles for more selective and
cautious opioid prescribing*
Principles for Patients Using Opioids Long-term
1.
2.
3.
4.
5.
Clearly communicate standardized expectations to
reduce risks
Adhere to recommended precautions
Avoid prescribing opioids and sedatives concurrently
Revisit discontinuing opioids or lowering dose
Identify and treat prescription opioid misuse disorders
* These principles are not intended for palliative care of chronic pain at end of life.
34
Principles for more selective and
cautious opioid prescribing*
Prepared by the faculty of the National Summit for
Opioid Safety
The National Summit had support from the Group
Health Foundation. It was co-sponsored by Group
Health Research Institute; Project ROAM (Dept. of
Family Medicine, University of Washington); and
Physicians for Responsible Opioid Prescribing (PROP).
* These principles are not intended for palliative care of chronic pain at end of life.
35
1/17/2013
36
Red Flags
Five (5) or more psychotropic medications prescribed
concomitantly (reviewed August 2012)
Two (2) or more concomitant antidepressants (reviewed October
2013)
Two (2) or more concomitant antipsychotic medications
(current)
Two(2) or more concomitant stimulant medications
long-acting plus short-acting ok
Three (3) or more concomitant mood stabilizer medications
Psychotropic polypharmacy (2 or more agents) for a given
mental disorder prescribed before utilizing psychotropic
monotherapy
37
Implementation of Red Flags
Retroactive
Evaluation
Identify
outliers
Profile
Review
DUR Board
Intervention
Reevaluation
Point of
service edits
• Targeted
education
• individuals
• overall
• Informational
(soft) –
pharmacist
override
• Hard Stop
Further
Action
38
40%
35%
Percent of Foster and Non-Foster Children Psychotropics by
36%
Drug Class
Calendar Year 2011
Total foster =2785
Total Non-Foster = 106,024
30%
25%
23%
21%
% Foster Children
20%
% Non-foster Children
15%
13%
10%
9%
6%
5%
4%
0%
0%
ADHD Drugs
Anti-depressants
Mood Stabilizers
Atypical Antipsychotics
39
40
Study Parameters and Results
Children in Foster Care ages 0-17
Time Period 4/1/2012 through 9/30/2012
49 patients were identified with fills for two or more
different antipsychotics during time period
26 patients received > or = 60 days concurrently
Other patients received for limited time period (1-2 fills)
or sequentially
41
Number of Participants by Age and
Sex Meeting Criteria
Number of Participants by Age and Sex
4.5
4
3.5
3
2.5
2
1.5
1
0.5
0
4M 4F 5M 5F 6M 6F 7M 7F 8M 8F 9M 9F 10M 10F 11M 11F 12M 12F 13M 13F 14M 14F 15M 15F 16M 16F 17M 17F
42
Diagnosis for Children Meeting Red Flag Threshold
Borderline Personality Disorder
Schizophrenic/Psychosis Disorders
Depressive Disorders
Pervasive Development Disorder
Autistic Disorder
All children had multiple diagnoses
Posttraumatic Stress Disorder
Disorders of Impulse Control
Conduct Disorder/Disturbance
Anxiety Disorders
Adjustment Reaction/Disorder
Oppositional Defiant Disorder
Reactive Attachment Disorder
Bipolar/Episodic Mood Disorders
ADHD
0
5
10
15
20
25
Number of Children
43
Prescriber Characteristics
Number of Prescribers per patient (antipsychotics only)
• Single prescriber
18 (65%)
• Two prescribers
6 (23%)
• Three prescribers
3 (12%)
Number of Patients Meeting Criteria/Prescriber
• Single patient = 21
• 2 patients = 1
• 3 patients = 2
• 4 patients = 1
• 5 patients = 1
44
Drug Combinations in Patients
Meeting Red Flag Threshold
risperidone and ziprasidone
quetiapine and risperidone
olanzapine and risperidone
olanzapine and quetiapine and risperidone
aripiprazole and ziprasidone
aripiprazole and risperidone and ziprasidone
aripiprazole and risperidone
aripiprazole and quetiapine
aripiprazole and olanzapine and risperidone
aripiprazole and haloperidol
airpiprazole and chlorpromazine
0
1
2
3
4
5
6
7
8
9
Number of Patients
45
46
Synagis Update
Idaho Medicaid’s outpatient prescription drug program
authorized payment for eligible patients for the 2012-2013
RSV season as of December 1, 2012.
Many hospitals started dosing Synagis in November 2012.
Doses given in the hospital are subtracted from the total
doses approved by Idaho Medicaid outpatient prescription
drug program.
AAP recommends a maximum of five monthly doses –
recommend utilizing Idaho specific epidemiology to
maximize drug benefit. After the fifth dose of Synagis,
most patients will have adequate RSV antibody titers for six
to seven weeks. The antibody levels do not plummet to
zero thirty days after the fifth Synagis dose.
47
Synagis Update
Season Start Dates
Dec 11
2005
Dec 21
2003
Dec 22
2008
Dec 31
2007
Jan 3
2010
Jan 9
2004
Jan 11
2009
Jan 13
2001
Jan 14
2000
Jan 15
2006
Jan 19
2002
Jan 21
2011
48
Synagis Update
Season End Dates
Mar 28
2004
Apr 10
2006
Apr 13
2003
Apr 24
2005
May 5
2008
May 6
2001
May 7
2011
May 9
2010
May 12
2002
May 19
2012
May 24
2009
May 28
2007
49
Synagis Update
In Idaho, Respiratory Syncytial Virus (RSV) season
officially began the week ending December 8, 2012.
The definition for season onset is adapted from the
National Respiratory and Enteric Virus Surveillance
System (NREVSS). RSV is considered widespread in
Idaho in the first of two consecutive weeks during
which the reported total percent of specimens testing
positive for antigen is ≥ 10%.
50
Revatio Use in Children
On August 30, 2012, the U.S. Food and Drug
Administration (FDA) sent out a safety announcement
recommending against the use of Revatio in children
with pulmonary hypertension. (handout in packet)
Revatio claims in Idaho Medicaid patients were
reviewed prior to and after the announcement for
comparison.
51
Revatio Use in Children
6/1/2012- 8/31/2012
10/1/2012 – 12/31/2012
12 claims
5 claims
5 patients
3 patients
$11,368
$4,015
3 patients continued from pre-announcement
Zero new patients post-announcement
52
Revatio Use in Children
Revatio became available generically as sildenafil 20mg
tablets in November 2012.
Five different generic manufacturers.
WAC brand (wholesale acquisition cost) - $20.41
WAC generic (wholesale acquisition cost) – as low as
$1.16
53
Proposed Studies for Next Quarter:
P&T Committee Narcotic Analgesic Studies – Next Steps
Use of Psychotropic Medications in Foster Children – Next
Steps
Two(2) or more concomitant stimulant medications
long-acting plus short-acting ok
Migraine Prevention
Prophylaxis Utilization in Chronic Triptan Utilizers
Botulinumtoxin Products
Testosterone enanthate
Testosterone cypionate
Antipsychotic Indication Evaluation- Hold for Future
AAP and DVTs- Hold for future
54
P&T Committee Narcotic Analgesic
Studies – Next Steps
55
Use of Psychotropic Medications in
Foster Children
The U.S. Government Accountability Office released
the results from a study that they performed
examining the rates of psychotropic medications for
foster and nonfoster children in 2008.
It was determined that HHS Guidance Could Help
States Improve Oversight of Psychotropic
Prescriptions.
56
40%
35%
Percent of Foster and Non-Foster Children Psychotropics by
36%
Drug Class
Calendar Year 2011
Total foster =2785
Total Non-Foster = 106,024
30%
25%
23%
21%
% Foster Children
20%
% Non-foster Children
15%
13%
10%
9%
6%
5%
4%
0%
0%
ADHD Drugs
Anti-depressants
Mood Stabilizers
Atypical Antipsychotics
57
Use of Psychotropic Medications in
Foster Children: Next Steps
Two(2) or more concomitant stimulant
medications
long-acting plus short-acting ok
58
Migraine Prevention
Prophylaxis Utilization in Chronic Triptan Utilizers
See packet for summary handout
59
Botulinumtoxin Products
Botulinumtoxin products are excluded from coverage by
the outpatient pharmacy prescription drug program –
these medications are only administered by health care
professionals and are not safe for patients to pick up and
“brown bag” to the doctor’s office.
Botulinumtoxin products are currently payable without
prior authorization on the medical side using J codes.
60
Botulinumtoxin Products
Totals
Trade
Name
# claims 12/01/2011 –
11/30/2012
$ for claims 12/01/2011 –
11/30/2012
Botox
478
$405,615
Dysport
21
$14,286
Myobloc
23
$11,133
Xeomin
3
$647
525
$431,681
61
Botulinumtoxin Products
Will review profiles of patients with paid claims on the
medical side to assess what the botulinumtoxin is most
likely being used for (e.g. cervical dystonia, migraines).
Even though Botox does not require prior authorization at
this time, the department has been receiving prior
authorization requests for Botox for migraines. Need to
develop criteria for Botox’s place in therapy as it is not firstline therapy. FDA approved for chronic migraines for
patients with at least 15 days of migraines per month with
each migraine lasting at least four hours.
62
Testosterone Products
Testosterone enanthate
Testosterone cypionate
63
Antipsychotic Indication EvaluationHold for Future
64
AAP and DVTs- Hold for future
65
Prospective DUR Report
History Errors:
• DD – drug-to-drug
• PG – drug to pregnancy
• TD – therapeutic duplication
• ER – early refill
• MC – drug-to-disease
Non-History Errors:
• PA – drug-to-age
• HD – high dose
• LD – low dose
• SX – drug-to-gender
66
Prospective DUR Report
Idaho Medicaid Program
ProDUR Message Report
December-12
ProDUR
Message
Drug To Drug
Drug To Gender
Drug To Known Disease
Drug To Pregnancy
Duplicate Therapy
Min Max
Too Soon Clinical
ALL
ProDUR
Severity
1
2
3
1
2
1
2
3
1
2
A
B
C
D
X
0
0
0
Message
Count
1,926
14,152
70,762
155
73
63,381
240,698
299,645
74
18
7
98
224
42
55
116,382
31,735
21,475
Message
Amount
$449,951.28
$2,627,612.23
$11,968,147.90
$38,527.92
$2,738.48
$8,079,221.73
$40,079,557.61
$49,081,346.38
$1,358.33
$170.80
$63.38
$20,479.73
$16,355.88
$6,954.37
$2,766.16
$22,561,821.67
$6,183,543.49
$3,922,520.03
860,902
$145,043,137.37
Total Number of Claims with Messages 211,935
Average ProDUR Message Per Claim
4.06
67
DUR Winter Newsletter
Copy of Fall Newsletter in packet
Brainstorm for new topics
68
Medicaid Update
69