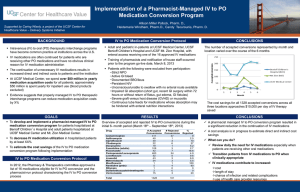
Informed Consent
advertisement

California Association of Health Facilities RAP Session San Bernardino/Riverside District Offices Presenters: Debra Brown, PharmD, Pharmaceutical Consultant Edwin Hoffmark, RN, Chief of RN Unit State of California Department of Public Health Licensing and Certification Program Debra.Brown@cdph.ca.gov RNUnit@cdph.ca.gov Welcome ! Please keep in mind that this presentation will not be all inclusive regarding CDPH Regulations Relevant questions are encouraged after the presentation. It is very important for everyone to remember that survey regulations (State and Federal) are the minimum standards and the facility should always strive for levels above this minimum. Goals and Objectives Identify Federal and State regulatory websites Summarize SNF pharmaceutical services Discuss statistical patterns of federal survey deficiencies related to SNF pharmaceutical services for 2010 and January – June 2011 Discuss Health and Safety Code 1261.6 related to use of Automated Drug Cabinets in SNFs Discuss elements of SNF Informed Consent for psychotherapeutic medications Helpful Federal SNF Regulatory Websites Federal Government Code of Federal Regulations (50) & U.S.C. http://www.gpoaccess.gov/cfr/ Use retrieve by CFR Title 42§§483 www.cms.hhs.gov - General Consumer Info. Skilled Nursing Regulations with Interpretive Guidelines & Appendix P http://www4.cms.hhs.gov/GuidanceforLawsAnd Regulations/12_NHs.asp Helpful California State Regulatory Websites California CDPH Licensing and Certification Homepage http://www.dhs.ca.gov/lnc/default.htm California’s 29 Statutes: Health and Safety Code http://www.leginfo.ca.gov/calaw.html California’s 28 Regulations: Title 22 http://ccr.oal.ca.gov/ Pharmaceutical Services Dispensing, labeling, storage, administration and disposition of medications Timeliness of medication services Stop orders Monitoring of the medication distribution system (ordering, dispensing, storage and administering) Medication ordering process and record keeping Controlled substances accountability Provision of pharmaceutical consultant services Pharmaceutical Consultant Services Assist in the development, coordination, supervision and review of pharmaceutical services Devote sufficient hours during visit for the purpose of coordinating, supervising and reviewing pharmaceutical services Provide consultation on all aspects of pharmaceutical services Serve on the following committees: Pharmaceutical Services, Patient Care Policy and Infection Control Committee minutes must be maintained in the facility and indicate members present, date, length of meeting, subjects discussed and action taken Pharmaceutical Consultant Services DRR Requirement At least monthly Review of all drugs currently ordered Information concerning the resident’s condition as related to drug therapy Medication administration records Physician progress notes Nurses notes Laboratory test results Report, in writing, to Administrator and DON Deficiency Patterns For California Skilled Nursing Facilities 2010 F329 Drug regimen is free from unnecessary drugs (69/283 Rx deficiencies = 24.3%) F332 Medication error rates of 5% or more (23/283 Rx deficiencies = 8.1 %) F333 Residents free from significant medication errors (31/283 Rx deficiencies = 11%) F425 Facility provides drugs and biologicals (65/283 Rx deficiencies = 23%) F428 Resident’s drug regimen reviewed monthly by the pharmacist (31/283 Rx deficiencies = 11%) F431 Proper labeling of drugs and biologicals (64/283 Rx deficiencies = 22.6%) Deficiency Patterns for California Skilled Nursing Facilities January – June 2011 F329 Drug regimen is free from unnecessary drugs (38/140 Rx deficiencies = 27.1%) F332 Medication error rates of 5% or more (3/140 Rx deficiencies = 2.1 %) F333 Residents free from significant medication errors (8/140 Rx deficiencies = 5.7%) F425 Facility provides drugs and biologicals (35/140 Rx deficiencies = 25%) F428 Resident’s drug regimen reviewed monthly by the pharmacist (25/140 Rx deficiencies = 17.9%) F431 Proper labeling of drugs and biologicals (31/140 Rx deficiencies = 22.1%) Automated Drug Cabinets (ADCs) in Skilled Nursing Facilities Health and Safety Code Section 1261.5 Allows for 48 medications/16 doses of each in emergency supplies (amended 1/2010) Does not apply to an automated drug delivery system when the pharmacist controls access to the medications Health and Safety Code Section 1261.6 ADC = mechanical system that stores/dispenses/distributes medication ADC also collects, controls and maintains transaction information (for security/accuracy/accountability) Written transaction information must be readily available for review/inspection ADC access limited to facility/contracted personnel authorized to administer medications Automated Drug Cabinets (ADCs) in Skilled Nursing Facilities (cont’d) Health and Safety Code Section 1261.6 Written P/P must be developed/implemented by the pharmacy and facility Ensure safety, accuracy, accountability, security, patient confidentiality, and maintenance of quality/potency/purity of stored medications P/P must be maintained at the pharmacy and the facility where ADC in use ADC also collects, controls and maintains transaction information (for security/accuracy/accountability) Automated Drug Cabinets (ADCs) in Skilled Nursing Facilities (cont’d) Health and Safety Code Section 1261.6 When used as an emergency medication supply container, drugs removed shall be limited to: A new drug order prescribed to a facility patient prior to the next delivery, or 72 hours, whichever is less Medications will be retrieved after authorization of the pharmacist (pursuant to review of the order) Medications ordered on “as needed” basis, if utilization and retrieval subject to ongoing pharmacist review Medications that patient care policy/pharmaceutical service committee agrees are emergency/acute onset drugs Pharmacist must review the order within 48 hours of retrieval Automated Drug Cabinets (ADCs) in Skilled Nursing Facilities (cont’d) Health and Safety Code Section 1261.6 When used to provide routine pharmacy services: ADC medications must be in labeled units of packaging Medications will be retrieved after review and approval of the pharmacist (pursuant to review of the order) Pharmacy providing services controls ADC access Access controlled using identification/password system/biosensor ADC shall make complete/accurate record of transactions that includes all users and medications added/removed After pharmacist reviews order, licensed staff only has access to patient-specific medications ordered by MD Automated Drug Cabinets (ADCs) in Skilled Nursing Facilities (cont’d) Health and Safety Code Section 1261.6 ADC systems that allow licensed staff to have access to multiple medications/not patient specific in their design are allowed if: ADC has electronic/mechanical safeguards to ensure retrieved medications are specific to that patient Facility notifies the department in writing prior to utilizing the system Notification shall include (not limited to) info re: ADC design, personnel access, P/P covering staff training, storage/security and administration issues Department will review above and ensure adequate safeguards in place to ensure medications delivered appropriate to patient If facility not in compliance, department may revoke authorization Automated Drug Cabinets (ADCs) in Skilled Nursing Facilities (cont’d) Health and Safety Code Section 1261.6 Stocking of ADC shall be done by a pharmacist If ADC utilizes removable pockets, cards, drawers, etc., the stocking system may be done outside the facility and delivered if: The task of placing removable pockets, cards, etc. is performed by pharmacist/pharmacy intern/tech working under pharmacist Removable pockets, cards, etc. are transported between the pharmacy and facility in a secure tamper-evident container Facility/pharmacy have developed P/P to ensure that pockets, cards, etc. are properly placed into ADC SNF Informed Consent Presented By: Edwin Hoffmark, RN, Chief of RN Unit Brief History of Informed Consent 1992 to 2011 1992 – Title 22 Section 72528 Promulgated 1993 – Release of Pharmacy Training Bulletin PTB-93-4 2010 2nd Qtr- Promulgation of 72528 revision 2010 3rd Qtr – Review of past training 2011 – AFL 11-08 Definitions and Meanings For the purposes of this presentation: The only facility that is covered by Title 22 Section 72528 is the Skilled Nursing Facility. Patient = Resident Whenever Patient is mentioned, it will include Responsible Party if applicable. What is Informed Consent Informed consent is first and foremost a Patient Right. Informed consent is granted by a Patient or a person who may act as a Patient’s Representative (Responsible Party.) Informed consent may be withdrawn at any time by the Patient or the RP (if applicable). Informed consent is a process that does not end with a signature or the start of therapy. Informed Consent is NOT Informed consent is not automatic. Informed consent is not a form. Informed consent is not finished when consent is obtained. PERTINENT STATE REGULATIONS CCR Title 22, Division 5 72018.1: Definition of Consent 72052: Definition of Informed Consent 72082: Definition of Physical Restraint 72092: Definition of Psychotherapeutic Drug 72527: Patient Rights (includes consent) 72528: Informed Consent Requirements OTHER PERTINENT STATE LAWS and DOCUMENTS Health and Safety Code HSC 1418.8 (Eppel Act) HSC 1418.9 Other related references: Schloendorff v. Society of New York Hospital (NY Supreme Court, 1914) Cobbs v. Grant, (Calif. Supreme Court, 1972) AFL 09-12 (Reiterates provisions of H&S 1418.8) AFL 11-08 ( Informed Consent) AFL 11-31 (Q & A Regarding Informed Consent) Q&A’s 1. 2. 3. 4. 5. 6. 7. 8. Agents Psychotherapeutic Medications Antipsychotics Exceptions Devices Material Circumstances… Verification Miscellaneous Agents Not Allowed Title 22 Section 72528(b) and HSC 1418.9 Agents (cont.) Title 22 Section 72528(b) (b) The information material to a decision concerning the administration of a psychotherapeutic drug or physical restraint, or the prolonged use of a device that may lead to the inability of the patient to regain use of a normal bodily function shall include at least the following: (1) The reason for the treatment and the nature and seriousness of the patient's illness. (2) The nature of the procedures to be used in the proposed treatment including their probable frequency and duration. (3) The probable degree and duration (temporary or permanent) of improvement or remission, expected with or without such treatment. (4) The nature, degree, duration and probability of the side effects and significant risks, commonly known by the health professions. (5) The reasonable alternative treatments and risks, and why the health professional is recommending this particular treatment. (6) That the patient has the right to accept or refuse the proposed treatment, and if he or she consents, has the right to revoke his or her consent for any reason at any time. Agents (cont.) HSC 1418.9 If the attending physician and surgeon of a resident in a skilled nursing facility prescribes, orders, or increases an order for an antipsychotic medication for the resident, the physician and surgeon shall do both of the following: (1) Obtain the informed consent of the resident for purposes of prescribing, ordering, or increasing an order for the medication. Psychotherapeutic Medications What is a psychotherapeutic medication? Any medication used to control behavior or to treat thought disorder processes. It is the use of the medication and not the classification* (*except antipsychotics) Psychotherapeutic Medications Includes: Medications not normally thought of as psychotherapeutic medications. Beta blockers used to control aggressive behavior. Sleeping medications used to control behavior e.g.; climbing out of bed, wandering… Any antipsychotic medication Psychotherapeutic Medications Does NOT Include: Sleeping medications used for sleep. Diazepam used for muscle spasms Antipsychotics Defined in HSC 1418.9(c)(3) "Antipsychotic medication" means a medication approved by the United States Food and Drug Administration for the treatment of psychosis. Exceptions to Informed Consent Title 22 Section 72528(e)&(f) • Emergencies • Documentation that the patient doesn’t want to be informed of the risk v. benefits. • Informing patient would cause harm. Devices 1. Physical Restraints – Title 22 § 72082 2. The prolonged use of a device that may lead to the inability to regain use of a normal bodily function. The Department has not specified any specific device or device category that would meet the informed consent requirements. Material Circumstances… The phrase is found in 72528(d) … unless material circumstances or risks change. A "change in material circumstances" usually means a change in the patient's medical or psychosocial condition which indicates that a different treatment plan should be considered. "Material circumstances" in this context are determined by licensed healthcare practitioners who are responsible for initiating a new informed consent discussion about any proposed changes to treatment. Healthcare practitioners are required to provide sufficient material information for the patient to make an informed decision to agree to the new treatment plan, to choose an alternative treatment or to refuse treatment altogether. Verification Verification required by Title 22 Section 72528(c) …facility staff shall verify that the patient's health record contains documentation that the patient has given informed consent … 1. 2. Licensed Healthcare Practitioners Patient or Family Miscellaneous 1. 2. 3. 4. Admission issues Documentation Acute Care Hospitals Policies and Procedures Informed Consent “…In the communications process, you, as the physician providing or performing the treatment and/or procedure (not a delegated representative), should disclose and discuss with your patient:…This communications process, or a variation thereof, is both an ethical obligation and a legal requirement spelled out in statutes and case law in all 50 states… “ Copyright 1995-2011 American Medical Association Questions??? Please ask questions as directed by the facilitator of this session so that we can ensure an accurate and thorough response. Contact Information If you have further questions about Informed Consent please contact Edwin Hoffmark, Chief of RN Unit at: California Department of Public Health Licensing & Certification Program Fax: (916) 324-4820 Email: RNUnit@cdph.ca.gov Phone: 1-800-236-9747