Hypotension in Acute Decompensated Heart Failure: Findings from ASCEND-HF
Priyesh A. Patela, Gretchen Heizera, Phillip J. Schultea, Christopher M. O’Connorb, Kenneth Dicksteinc, Justin A. Ezekowitzd, Vic Hasselblada, Barry M. Massiee, Roger M. Millsf, John J. McMurrayg, Randall C. Starlingh,
W. H. Wilson Tangh, Robert M. Califfa,b, Adrian F. Hernandeza,b
aDuke
Clinical Research Institute, Durham, North Carolina, USA; bDuke University Medical Center, Durham, North Carolina, USA; cStavanger University Hospital, Stavanger, Norway; dDivision of Cardiology, Department of Medicine, University of Alberta, Edmonton, Canada;
eUniversity of California San Francisco and San Francisco Veterans Affairs Hospital, San Francisco, California, USA; fJanssen Research & Development, LLC, Raritan, New Jersey, USA; gInstitute of Cardiovascular and Medical Sciences, BHF Glasgow Cardiovascular
Research Centre, University of Glasgow, Glasgow, UK; hCleveland Clinic Foundation, Cleveland, Ohio, USA
Background
Methods
Results
Context: Transient, in-hospital hypotension
while hospitalized for acute decompensated
heart failure (ADHF) causes clinical concern, but
contributing factors and its association with
outcomes are poorly understood.
Study Population : 7,141 patients with ADHF were randomized to nesiritide or placebo within 24 hours of
first IV therapy. Clinical and laboratory data were recorded for all patients.
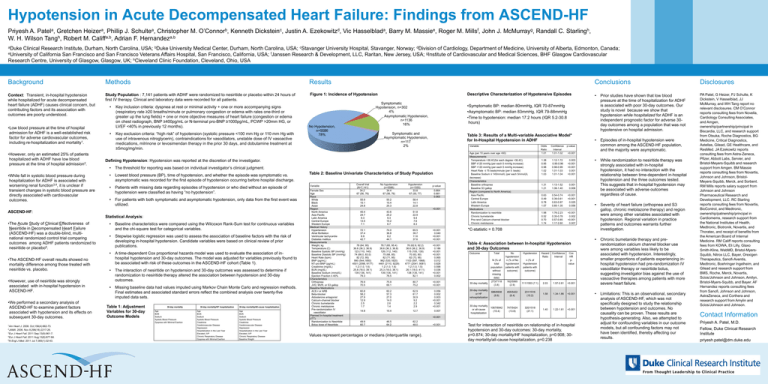
Figure 1: Incidence of Hypotension
•Low blood pressure at the time of hospital
admission for ADHF is a well-established risk
factor for adverse cardiovascular outcomes,
including re-hospitalization and mortality1.
•However, only an estimated 25% of patients
hospitalized with ADHF have low blood
pressure at the time of hospital admission2.
•While fall in systolic blood pressure during
hospitalization for ADHF is associated with
worsening renal function3,4, it is unclear if
transient changes in systolic blood pressure are
directly associated with cardiovascular
outcomes.
•
•
Key inclusion criteria: dyspnea at rest or minimal activity + one or more accompanying signs
(respiratory rate ≥20 breaths/minute or pulmonary congestion or edema with rales one-third or
greater up the lung fields) + one or more objective measures of heart failure (congestion or edema
on chest radiograph, BNP ≥400pg/mL or N-terminal pro-BNP ≥1000pg/mL, PCWP >20mm HG, or
LVEF <40% in previously 12 months).
Key exclusion criteria: “high risk” of hypotension (systolic pressure <100 mm Hg or 110 mm Hg with
use of intravenous nitroglycerin), contraindications for vasodilators, unstable dose of IV vasoactive
medications, milrinone or levosimendan therapy in the prior 30 days, and dobutamine treatment at
≥5mcg/mg/min.
•
The threshold for reporting was based on individual investigator’s clinical judgment.
•
Lowest blood pressure (BP), time of hypotension, and whether the episode was symptomatic vs.
asymptomatic was recorded for the first episode of hypotension occurring before hospital discharge.
•
Patients with missing data regarding episodes of hypotension or who died without an episode of
hypotension were classified as having “no hypotension”.
•
For patients with both symptomatic and asymptomatic hypotension, only data from the first event was
utilized.
Statistical Analysis:
•
•
•
•The ASCEND-HF overall results showed no
mortality difference among those treated with
nesiritide vs. placebo.
•However, use of nesiritide was strongly
associated with in-hospital hypotension in
ASCEND-HF.
•We performed a secondary analysis of
ASCEND-HF to examine patient factors
associated with hypotension and its effects on
subsequent 30-day outcomes.
1Am
Heart J. 2008. Oct;156(4):662-73.
2JAMA. 2006. Nov 8;296(18):2217-26.
3Eur J Heart Fail. 2011 Sep;13(9):961-7.
4Eur J Heart Fail..2011 Aug;13(8):877-84.
5N Engl J Med. 2011 Jul 7;365(1):32-43.
Symptomatic
Hypotension, n=302
4%
Asymptomatic Hypotension,
n=1136
16%
No Hypotension,
n=5586
78%
Symptomatic and
Asymptomatic Hypotension,
n=117
2%
Table 3: Results of a Multi-variable Associative Model*
for In-Hospital Hypotension in ADHF
Variable
•
•
Baseline characteristics were compared using the Wilcoxon Rank-Sum test for continuous variables
and the chi-square test for categorical variables.
Stepwise logistic regression was used to assess the association of baseline factors with the risk of
developing in-hospital hypotension. Candidate variables were based on clinical review of prior
publications.
A time-dependent Cox proportional hazards model was used to evaluate the association of inhospital hypotension and 30-day outcomes. The model was adjusted for variables previously found to
be associated with risk of these outcomes in the ASCEND-HF cohort (Table 1).
The interaction of nesiritide on hypotension and 30-day outcomes was assessed to determine if
randomization to nesiritide therapy altered the association between hypotension and 30-day
outcomes.
Missing baseline data had values imputed using Markov Chain Monte Carlo and regression methods.
Final estimates and associated standard errors reflect the combined analysis over twenty-five
imputed data sets.
Table 1: Adjustment
Variables for 30-day
Outcome Models
30-day mortality
Age
BUN
Sodium
Systolic Blood Pressure
Dyspnea with Minimal Exertion
30-day mortality/HF hospitalization
Age
BUN
Sodium
Systolic Blood Pressure
Creatinine
Cerebrovascular Disease
Depression
Hospitalization in the Last Year
Elevated JVP
Chronic Respiratory Disease
Dyspnea with Minimal Exertion
30-day mortality/All-cause hospitalization
Age
BUN
Sodium
Systolic Blood Pressure
Creatinine
Cerebrovascular Disease
Depression
Hospitalization in the Last Year
Elevated JVP
Chronic Respiratory Disease
Baseline Weight
Table 2: Baseline Univariate Characteristics of Study Population
Variable
Female Sex
Age
Race
White
Black
Asian
Region
North America
Asia Pacific
Latin America
Central Europe
Western Europe
Medical History
Hypertension
Atrial fibrillation
Ventricular tachycardia
Diabetes mellitus
Measurements
Weight, kg
Temperature (C)
Baseline Systolic BP (mmHg)
Baseline Diastolic BP (mmHg)
Heart Rate (bpm)
BNP (pg/mL)
NT-pro-BNP (pg/mL)
Creatinine (mg/dL)
BUN (mg/dL)
Baseline Sodium (mmol/L)
Ejection Fraction ≤ 40%
Characteristics
Orthopnea
JVD, MVR, or S3 gallop
Baseline Medications
ACE-I or ARB
Beta-blocker
Aldosterone antagonist
Calcium-channel blocker
Chronic bumetanide
Chronic metolazone
Pre-randomization IV
vasodilator
Planned In-hospital treatment
(ITT)
Randomization to Nesiritide
Bolus dose of Nesiritide
Overall trial
(N=7,141)
34.2
67 (56, 76)
No hypotension
(n=5586)
34.4
67 (56, 76)
Hypotension
(n=1555)
33.8
67 (55, 77)
55.9
15.1
24.8
55.2
15.4
25.3
58.4
14.1
22.8
45.4
24.7
9.3
13.5
7.1
43.6
25.2
9.3
15.2
6.7
51.8
22.9
9.4
7.6
8.3
72.1
37.4
8.9
42.7
74.0
36.8
8.2
44.1
65.5
39.7
11.6
37.6
p-value
0.664
0.681
0.063
<0.001
<0.001
0.040
<0.001
<0.001
Age (per 10 years over age >60)
Measurements
Temperature >36.4C(for each degree >36.4C)
SBP ≤120 mmHg (per each 5 mmHg increase)
SBP >120 mmHg (per each 5 mmHg increase)
Heart Rate ≤ 75 beats/minute (per 5 beats)
Baseline Sodium ≤ 145mmol/L (per each 5mmol)/L
decrease)
Characteristics
Baseline orthopnea
Baseline S3 gallop
Region (compared to North America)
Asia Pacific
Central Europe
Latin America
Western Europe
Medications
Randomization to nesiritide
Chronic bumetanide
Pre-rand Calcium-channel blocker
Chronic metolazone
76 (62.6, 92.2)
36.6 (36.2, 36.9)
115 (105, 130)
70 (63, 80)
82 (73, 95)
1103 (597, 1968)
4771 (2041, 9081)
1.2 (1.0, 1.5)
26.1 (18.0, 41.1)
138 (135, 141)
82.1
<0.001
0.186
<0.001
<0.001
0.065
0.012
0.828
0.598
0.036
<0.001
0.002
76.9
70.5
75.9
69.1
80.7
75.2
<0.001
<0.001
30-day mortality
60.8
58.2
27.9
12.9
2.5
1.7
60.2
57.3
27.0
14.0
2.6
1.4
62.9
61.7
30.9
9.2
2.2
2.9
0.056
0.002
0.003
<0.001
0.342
<0.001
30-day mortality
or HF
rehospitalization
14.8
15.4
12.7
0.007
30-day mortality
or all-cause
hospitalization
Odds
Ratio
1.01
Confidence
Interval
1.01-1.02
p-value
Values represent percentages or medians (interquartile range).
•
Episodes of in-hospital hypotension were
common among the ASCEND-HF population,
and the majority were asymptomatic.
•
While randomization to nesiritide therapy was
strongly associated with in-hospital
hypotension, it had no interaction with the
relationship between time-dependent in-hospital
hypotension and the three outcomes tested.
This suggests that in-hospital hypotension may
be associated with adverse outcomes
regardless of cause.
•
Severity of heart failure (orthopnea and S3
gallop, chronic metolazone therapy) and region
were among other variables associated with
hypotension. Regional variation in practice
patterns and outcomes warrants further
investigation.
•
Chronic bumetanide therapy and prerandomization calcium channel blocker use
were among variables less likely to be
associated with hypotension. Interestingly,
smaller proportions of patients experiencing inhospital hypotension had pre-randomization IV
vasodilator therapy or nesiritide bolus,
suggesting investigator bias against the use of
vasoactive therapies among patients with more
severe heart failure.
•
Limitations: This is an observational, secondary
analysis of ASCEND-HF, which was not
specifically designed to study the relationship
between hypotension and outcomes. No
causality can be proven. These results are
hypothesis-generating. Also, we attempted to
adjust for confounding variables in our outcome
models, but all confounding factors may not
have been identified, thereby affecting our
results.
PA Patel, G Heizer, PJ Schulte, K
Dickstein, V Hasselblad, JJ
McMurray, and WH Tang report no
relevant disclosures. CM O’Connor
reports consulting fees from Novella,
Cardiology Consulting Associates,
and Amgen,
ownership/partnership/principal in
Biscardia, LLC, and research support
from Otsuka, Roche Diagnostics, BG
Medicine, Critical Diagnostics,
Astellas, Gilead, GE Healthcare, and
ResMed. JA Ezekowitz reports
consulting fees from Astra-Zeneca,
Pfizer, Abbott Labs, Servier, and
Bristol-Meyers-Squibb and research
support from Amgen. BM Massie
reports consulting fees from Novartis,
Johnson and Johnson, BristolMeyers-Squibb, Merck, and Sorbent.
RM Mills reports salary support from
Johnson and Johnson
Pharmaceutical Research and
Development, LLC. RC Starling
reports consulting fees from Novartis,
BioControl, and Medtronic,
ownership/patnership/principal in
Cardiomems, research support from
the National Institutes of Health,
Medtronic, Biotronik, Novartis, and
Thoratec, and receipt of benefits from
the American Board of Internal
Medicine. RM Califf reports consulting
fees from KOWA, Eli Lilly, Glaxo
Smith-Kline, WebMD, Bristol-MyersSquibb, Nitrox LLC, Bayer, Orexigen
Therapeutics, Sanofi-Aventis,
Medtronic, Boehringer Ingelheim, and
Gilead and research support from
BMS, Roche, Merck, Novartis,
Scios/Johnson and Johnson, Amilyn,
Bristol-Myers-Squibb, and Bayer. AF
Hernandez reports consulting fees
from Sanofi, Johnson and Johnson,
AstraZeneca, and Corthera and
research support from Amylin and
Scios/Johnson and Johnson.
1.38
0.95
0.98
1.02
1.03
1.12-1.72
0.95-0.96
0.98-0.99
1.01-1.03
1.01-1.04
0.003
<0.001
<0.001
0.001
<0.001
1.31
1.21
1.13-1.52
1.06-1.40
0.001
0.006
0.63
0.49
0.78
1.07
0.54-0.74
0.39-0.61
0.63-0.97
0.85-1.35
<0.001
<0.001
0.025
0.556
1.98
0.52
0.70
1.74
1.76-2.23
0.35-0.78
0.57-0.85
1.17-2.60
<0.001
0.002
<0.001
0.007
<0.001
Outcome
Total
N (% of
total
population
without
missing
data)
273/7118
(3.8)
No
hypotension
n (% of No
hypotension
patients with
outcome)
Hypotension
Hazard
Ratio
Confidence
Interval
Cox
HR
pvalue
n (% of
Hypotension
patients with
outcome)
162/5565
(2.9)
111/1553 (7.1)
2.03
1.57-2.61
<0.001
686/6938
(9.9)
455/5422
(8.4)
231/1516
(15.2)
1.58
1.34-1.86
<0.001
1067/6942
(15.4)
747/5424
(13.8)
320/1518
(21.1)
1.40
1.22-1.61
<0.001
<0.001
62.2
48.0
Prior studies have shown that low blood
pressure at the time of hospitalization for ADHF
is associated with poor 30-day outcomes. Our
study is novel because we show that
hypotension while hospitalized for ADHF is an
independent prognostic factor for adverse 30day outcomes among a population that was not
hypotensive on hospital admission.
Table 4: Association between In-hospital Hypotension
and 30-day Outcomes
78.7 (65, 95.4)
36.6 (36.3, 36.8)
126 (112, 140)
75 (68, 85)
82 (71, 95)
962 (530,1820)
4461 (2110, 9229)
1.2 (1.0, 1.6)
25.3 (18.0, 38.1)
139 (136, 141)
78.5
46.5
64.2
•
*C-statistic = 0.708
78 (64, 95)
36.6 (36.3, 36.9)
123 (110, 140)
74 (66.0, 83.0)
82 (72, 95)
990 (544,1850)
4501 (2098, 9177)
1.2 (1.0, 1.6)
25.8 (18.0, 39.1)
139 (136, 141)
79.3
49.9
60.7
Disclosures
•Symptomatic BP: median 80mmHg, IQR 70-87mmHg
•Asymptomatic BP: median 83mmHg, IQR 79-88mmHg
•Time to hypotension: median 17.2 hours (IQR 5.2-30.8
hours)
Defining Hypotension: Hypotension was reported at the discretion of the investigator.
ASCEND-HF:
•The Acute Study of Clinical Effectiveness of
Nesiritide in Decompensated Heart Failure
(ASCEND-HF) was a double-blind, multinational randomized control trial comparing
outcomes among ADHF patients randomized to
nesiritide or placebo5.
Descriptive Characterization of Hypotensive Episodes
Conclusions
<0.001
Test for interaction of nesiritide on relationship of in-hospital
hypotension and 30-day outcomes: 30-day mortality,
p=0.874; 30-day mortality/HF hospitalization, p=0.908; 30day mortality/all-cause hospitalization, p=0.238
Contact Information
Priyesh A. Patel, M.D.
Fellow, Duke Clinical Research
Institute
priyesh.patel@dm.duke.edu










