Atoms and Molecules
advertisement

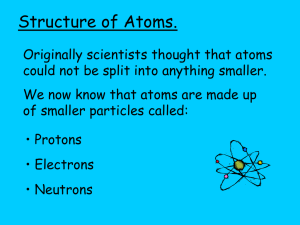
Atoms and Molecules Atom is smallest particle that can be identified as a particular substance. Molecule is two or more atoms bonded to each other. Elements Around 110 different elements have been identified. Of these around 80 are found in nature on Earth. Most common element on Earth is oxygen, symbol O. Most common element in universe is hydrogen, symbol H. Symbols Symbols are shorthand for the name of the element. Usually first one or two letters of name. Atoms Atom is the basic unit of an element. Atoms contain protons, neutrons, and electrons. Protons – positive charge (+1) Neutrons – neutral, no charge Electrons – negative charge (-1) Nucleus Contains protons and neutrons. All positive charge of atom is in nucleus. Majority of mass is in nucleus. Protons have mass of 1 amu. Neutrons have mass of 1 amu. Electrons Mass is about 1/2000 of the mass of a proton. Electrons orbit the nucleus similarly to planets orbiting the sun. All negative charge of the atom is due to electrons. Charge of the atoms Elements have no net charge. The number of protons equals the number of electrons. The Subatomic Particles Proton – the identity particle. The number of protons in the nucleus determines which element the atom is. Neutrons – a neutral particle Electrons – the personality particle. The electrons give the atom its characteristic behaviors. The Symbol Box The Atomic Number – usually at top of box. Is the number of protons in the nucleus. In an element, the atomic number is also the number of electrons. The Symbol – shorthand for name. The Atomic Mass – usually at the bottom of box. Is the sum of the protons and neutrons. Masses AMU – atomic mass unit. 1.67 x 10-24 g Proton and neutron 1 AMU Election mass – 9.11 x 10-28 g Carbon atom: mass = 12 amu Gain/Loss of Electrons Atoms can gain from other atoms or lose electrons to other atoms. An atom that loses an electron has a net positive charge because there are more protons than electrons. Remember the atom started with equal numbers of protons and electrons. An atom that gains an electron has a net negative charge because there are more electrons than protons. Ions When an atom has lost or gained an electrons it is called an ion. Ions with positive charge are known as CATIONS Ions with negative charge are known as ANIONS The Periodic Table An organized table of all known elements. To the left of the stairstep are metals To the right of the stairstep are nonmetals Along both sides of the stairstep are metalloids. Rows are known as periods Columns are known as groups Neutrons Isotopes To be a particular element, an atom must have a specific number of protons, i.e. 8 protons are in an oxygen atom. Most commonly an oxygen atom has 8 neutrons. Sometimes the nucleus contains has a different number of neutrons. Isotope is the term for atoms of a single element that have different numbers of neutrons. Specifying an Atom See paper on symbols on www.drbillboyle.com