Rate Laws
advertisement

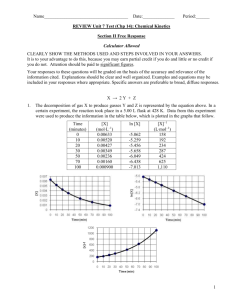
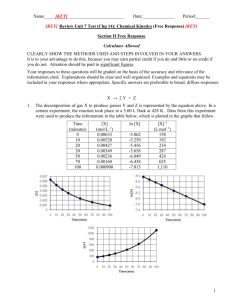
Rate Laws Rate Laws Rate = k [A]x [B]y [A] and [B] represent the concentrations of reactants x and y often (but not always) represent the coefficients in front of [A] and [B] k is a constant specific to the reaction. Rate Laws Rate = k [A]x [B]y k A constant specific to the reaction. Rate constants may have a variety of strange units using L, mol, and sec. This is so the rate will always work out in mol/L sec. Rate Laws For example: 2NO + O2 2 NO2 The rate expression is: Rate = k [NO]2[O2] Rate Laws For example: 2 N2O5 4 NO2 + O2 The rate expression is Rate = k [N2O5]2 Rate Laws Order in reactions: refers to the exponents on the compounds in the rate law Rate = k [NO]2[O2] This reaction is second order for NO and first order for O2 Rate Law This rate expression Rate = k [NO]2[O2] is third order overall. Rate Laws If a reactant is first order, doubling the concentration will double the rate. If a reactant is second order, doubling the concentration will quadruple the rate. If changing the concentration has no effect the chemical does not appear in the rate law. Rate Laws To determine the rate constant, one must collect data using different concentrations of reactants. Rate Laws For example: H2O2 + 2HI 2H2O + I2 Trial [H2O2] [HI] 1 0.1 M 0.1 M 2 0.1 M 0.2 M 3 0.2 M 0.1 M Rate .0076 .0152 .0152 Rate Laws If [HI] doubles, the rate doubles, so the rate expression is first order for HI. If [H2O2] doubles, the rate doubles, so the rate expression is first order for H2O2. Rate Laws The rate expression must be Rate = k [H2O2] [HI] Rate Laws Plug in data from any trial to solve for k: .0076 = k (0.1M) (0.1M) .0076 = .01k K = .76 Rate Laws If a reaction has two or more steps, the rate-determining step is the slowest one. Rate Laws Rate-determining Step 2 NO(g) + 2H2(g) N2(g) + 2 H2O(g) Elementary Steps 2 NO N2O2 (fast) N2O2 + H2 N2O + H2O + H2 (slow) N2O + H2 N2 + H2O (fast) Rate Laws Rate-determining Step Which is the rate-determining step? 2 NO N2O2 (fast) N2O2 + H2 N2O + H2O + H2 (slow) N2O + H2 N2 + H2O (fast) ____________________________________________________________________________________ 2 NO(g) + 2H2(g) N2(g) + 2 H2O(g) Rate Laws Rate-determining Step Which is the rate-determining step? 2 NO N2O2 (fast) N2O2 + H2 N2O + H2O + H2 (slow) N2O + H2 N2 + H2O (fast) ____________________________________________________________________________________ 2 NO(g) + 2H2(g) N2(g) + 2 H2O(g)