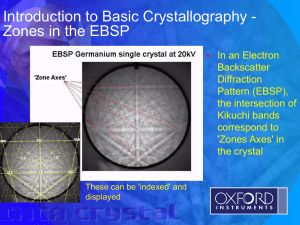
Crystallography
advertisement

Introduction to Crystallography and Mineral Crystal Systems Prof. Dr. Andrea Koschinsky Geosciences and Astrophysics Why Crystallography in Geosciences? • Most of the Earth is made of solid rock. The basic units from which rocks are made are minerals. • Minerals are natural crystals, and so the geological world is largely a crystalline world. • The properties of rocks are ultimately determined by the properties of the constituent minerals, and many geological processes represent the culmination - on a very grand scale - of microscopic processes inside minerals. • For example, large-scale processes, such as rock formation, deformation, weathering and metamorphic activity, are controlled by small-scale processes, such as movement of atoms (diffusion), shearing of crystal lattices (dislocation movement), growth of new crystals (nucleation, crystallization), and phase transformations. Why Crystallography in Geosciences? • An understanding of mineral structures and properties also allows us to answer more immediate questions, such as why quartz and diamond are so hard, and why solid granite rock is destined to become soft, sticky clay. • Minerals are natural resources, providing raw materials for many industries. Therefore, understanding minerals has geological as well as economic applications. • Definition of the term “MINERAL”: a solid body, formed by natural processes, that has a regular arrangement of atoms which sets limits to its range of chemical composition and gives it a characteristic crystal shape. Definition of Crystallography • CRYSTALLOGRAPHY is the study of crystals. • CRYSTALLOGRAPHY is a division of the entire study of mineralogy. • Geometrical, physical, and chemical CRYSTALLOGRAPHY • A CRYSTAL is a regular polyhedral form, bounded by smooth faces, which is assumed by a chemical compound, due to the action of its interatomic forces, when passing from the state of a liquid or gas to that of a solid. • Polyhedral form: solid bounded by flat planes (CRYSTAL FACES). • Very slow cooling of a liquid allows atoms to arrange themselves into an ordered pattern, which may extend of a long range (millions of atoms). This kind of solid is called crystalline. • Example: The chemical composition of window glass is virtually identical with that of quartz (a crystalline material): both are forms of SiO2. Window glass is glassy because it is made by chilling molten SiO2 very quickly; quartz crystals form when molten SiO2 is cooled very slowly or by precipitation from solution. Crystal Forms • During the process of crystallization, crystals assume various geometric shapes dependent on the ordering of their atomic structure and the physical and chemical conditions under which they grow. • These forms may be subdivided, using geometry, into six systems. CRYSTALLOGRAPHIC AXES 6 large groups of crystal systems: • • • • • • (1) CUBIC (2) TETRAGONAL (3) ORTHORHOMBIC (4) HEXAGONAL (5) MONOCLINIC (6) TRICLINIC (1) CUBIC (aka ISOMETRIC) The three crystallographic axes a1, a2, a3 (or a, b, c) are all equal in length and intersect at right angles (90 degrees) to each other. (2) TETRAGONAL Three axes, all at right angles, two of which are equal in length (a and b) and one (c) which is different in length (shorter or longer). Note: If c was equal in length to a or b, then we would be in the cubic system! (3) ORTHORHOMBIC Three axes, all at right angles, and all three of different lengths. Note: If any axis was of equal length to any other, then we would be in the tetragonal system! (4) HEXAGONAL Four axes! Three of the axes fall in the same plane and intersect at the axial cross at 120 degrees between the positive ends. These 3 axes, labeled a1, a2, and a3, are the same length. The fourth axis, termed c, may be longer or shorter than the a axes set. The c axis also passes through the intersection of the a axes set at right angle to the plane formed by the a set. (5) MONOCLINIC Three axes, all unequal in length, two of which (a and c) intersect at an oblique angle (not 90 degrees), the third axis (b) is perpendicular to the other two axes. Note: If a and c crossed at 90 degrees, then we would be in the orthorhombic system! (6) TRICLINIC The three axes are all unequal in length and intersect at three different angles (any angle but 90 degrees). Note: If any two axes crossed at 90 degrees, then we would be describing a monoclinic crystal! MILLER INDICES Mathematical system for describing any crystal face or group of similar faces (forms) developed by William H. Miller (1801-1880). Face of an octahedron using Miller's indices: An octahedron is an eight-sided crystal form that is the simple repetition of an equilateral triangle about our 3 crystallographic axes. The triangle is oriented so that it crosses the a1 (or a), a2 (or b), and a3 (or c) axes all at the same distance from the axial cross. This unit distance is given as 1. So the Miller indices is (111) for the face that intercepts the positive end of each of the 3 axes. Note: A bar over the number tells me that the intercept was across the negative end of the particular crystallographic axis. Face of a cube using Miller's indices: A cube face that intercepts the a3 (vertical) axis on the + end will not intercept the a1 and a2 axes. If the face does not intercept an axis, then we assign a mathematical value of infinity to it. So we start with Infinity, Infinity, 1 (a1, a2, a3). So the Miller indices of the +a3 intercept face equals (001). ELEMENTS OF SYMMETRY • PLANES OF SYMMETRY Any two dimensional surface that, when passed through the center of the crystal, divides it into two symmetrical parts that are MIRROR IMAGES is a PLANE OF SYMMETRY A cube has 9 planes of symmetry, 3 of one set and 6 of another. In the left figure the planes of symmetry are parallel to the faces of the cube form, in the right figure the planes of symmetry join the opposite cube edges. • AXES OF SYMMETRY Any line through the center of the crystal around which the crystal may be rotated so that after a definite angular revolution the crystal form appears the same as before is termed an axis of symmetry. Depending on the amount or degrees of rotation necessary, four types of axes of symmetry are possible when you are considering crystallography: When rotation repeats form every 60 degrees, then we have sixfold or HEXAGONAL SYMMETRY. When rotation repeats form every 90 degrees, then we have fourfold or TETRAGONAL SYMMETRY. When rotation repeats form every 120 degrees, then we have threefold or TRIGONAL SYMMETRY. When rotation repeats form every 180 degrees, then we have twofold or BINARY SYMMETRY. • CENTER OF SYMMETRY. Most crystals have a center of symmetry, even though they may not possess either planes of symmetry or axes of symmetry. Triclinic crystals usually only have a center of symmetry. If you can pass an imaginary line from the surface of a crystal face through the center of the crystal (the axial cross) and it intersects a similar point on a face equidistance from the center, then the crystal has a center of symmetry. The crystal face arrangement symmetry of any given crystal is simply an expression of the internal atomic structure. The relative size of a given face is of no importance, only the angular relationship or position to other given crystal faces. CRYSTAL FORMS AND SYMMETRY CLASSES HABIT is the correct term to indicate outward appearance. Habit, when applied to natural crystals and minerals, includes such descriptive terms as tabular, equidimensional, massive, reniform, drusy, and encrusting. A FORM is a group of crystal faces, all having the same relationship to the elements of symmetry of a given crystal system. These crystal faces display the same physical and chemical properties because the ATOMIC ARRANGEMENT (internal geometrical relationships) of the atoms composing them is the same. Note: Crystals, even of the same mineral, can have differing CRYSTAL FORMS, depending upon their conditions of growth. Example: Various Crystal Forms of Peruvian Pyrite Pyrite is a common mineral which often exhibits several forms on a single crystal. One form is usually dominant, presenting the largest faces on the crystal. Peruvian pyrite commonly has cubic, octahedral, and dodecahedral forms on a single crystal. Crystals with the same forms present, but with different dominant forms will each appear very different. There are 32 forms in the nonisometric (noncubic) crystal systems and another 15 forms in the isometric (cubic) system. Introduction to the atomic arrangement of crystal forms Crystal Lattice Structures Simple Cubic and Related Structures Schematic diagram of an atom of the element carbon. The nucleus contains six protons and six neutrons. Electrons orbiting the nucleus are confined to specific orbits called energy-level shells. A. Three-dimensional representation showing the first energy-level shells. The first shell can contain two electrons, the second eight. B. B. Two-dimensional representation of the carbon atom to show the number of protons and neutrons in the nucleus and the number of electrons in the energy-level shells. The first energy-level shell is full because it contains two electrons. The second shell contains four electrons and so is half full. Model for the ionic compound LiF To form the compound lithium fluoride, an atom of the element lithium combines with an atom of the element fluorine. The lithium atom transfers its lone outer-shell electron to fill the fluorine atom's outer shell, creating an Li+ cation and a F- anion in the process. The electrostatic force that keeps the lithium and fluorine ions together in the compound lithium fluoride is an ionic bond. Mineral structure of PbS The arrangement of ions in the most common lead mineral, galena (PbS). Lead forms a cation with a charge of 2+, and sulfur forms an anion with a charge of 2-. To maintain a charge balance between the ions, there must be an equal number of Pb and S ions in the structure. The packing arrangement of ions is repeated continuously through a crystal. The ions are shown pulled apart along the black lines to demonstrate how they fit together. The tetrahedron-shaped silicate anion SiO4(-4) A. B. Anion with the four oxygens touching each other in natural position. Silicon (dashed circle) occupies central space. Exploded view showing the relatively large oxygen anions at the four corners of the tetrahedron, equidistant from the relatively small silicon cation. Polymerization of complex silicate anions Polymerization of complex silicate anions Summary of the way silicate anions polymerize to form the common silicate minerals. The most important polymerizations are those that produce chains, sheets, and three-dimensional networks. Snow Crystals Snow crystals: Individual ice crystals, often with six-fold symmetrical shapes. They grow directly from the condensing water vapor in the air, size microscopic to at most a few mm in diameter Snowflakes: Collections of snow crystals, loosely bound together into a puffball. Can grow to large sizes (up to 10 cm across) Plate forms: Simple sectored plate Dendritic sectored plate fern-like stellar dendrite Columns forms: Hollow column (sheet-like crystal) Needle crystal