12-1_Chemical_Reactions_That_Involve_Heat
advertisement

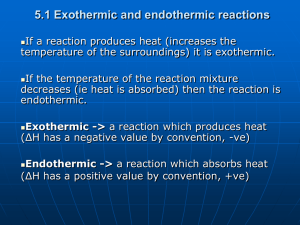
12-1 Chemical Reactions That Involve Heat Law of conservation of energy - energy is neither created or destroyed. Most chemical reactions involve changes in energy. The making and breaking of chemical bonds either absorb or release energy. Heat is the transfer of energy from one object to another. Temperature is a measure of kinetic energy Thermochemistry- the study of changes in heat in chemical reactions. Exothermic Reactions Release heat (exo=outside, thermic=heat) Combustion is an exothermic reaction. When you burn things heat is put out, you feel it! When reactions are exothermic, release heat, ENERGY is released. Therefore, the products have less energy than the reactants. Endothermic Reactions Reactions that absorb heat (endo=inside, thermic=heat). Chemical bonds formed store heat as energy. Energy must be supplied to make these reactions occur. Products have more energy than reactants because of energy stored in bonds. GUMMY BEAR SACRIFICE! http://vimeo.com/24012128 Is this an example of an endothermic or exothermic reactions? How do you know??? 12-1 Section Review Questions… 1. In an exothermic reaction, which side of the chemical equation would you write the term energy? 2. In an endothermic reaction, which side of the chemical equation would you write the term energy? 3. Is cloud formation exothermic or endothermic? Why do you feel cooler on a hot summer day when you mist yourself with water and let it evaporate off you? Hint: Who has more kinetic energysolids, liquids or gases? So is energy being lost or gained in each phase change? 4. Why do you think your body produces sweat? Why are you in trouble when you stop sweating (2 parts)? 12-2 Heat and Enthalpy Changes Energy is measured in kilojoules (kJ)! Enthalpy-depends on temperature, phase (state of matter), and composition. When pressure remains constant, the heat released or absorbed in a chemical reaction is equal to the enthalpy change. Enthalpy Change (delta ΔH) the heat gained or lost during a chemical reaction. The formula is: ΔH = enthalpy of products – enthalpy of reactants Enthalpy Change for Endothermic Reactions… ΔH for endothermic = positive Who has the higher energy the reactants or the products? ΔH for exothermic = negative Stoichiometry & Enthalpy! Solving enthalpy problems requires stoichiometry Observe the following reaction: 2H2O2(l) 2H2O(l) + O2(g) = -190kJ Is this rxn endo or exo thermic? Sample Enthalpy Problem How much heat is transferred when 9.22g of glucose in your body reacts with oxygen in respiration, which produces carbon dioxide and water. 1 mole of glucose delta H = -2803 kJ 9.22g glucose 1 mol glucose -2803kJ 180g glucose 1mol glucose = - 144 kJ Sample #2 147g of Nitrogen dioxide is dissolved in water to produce nitric acid and nitrogen monoxide. 3mol nitrogen dioxide delta H = –138kJ 3NO2 + H2O 2HNO3 + NO 147g NO2 1mol NO2 46g NO2 -138 kJ 3mol NO2 = - 147 kJ 12-3 Hess’s Law Hess’s Law –The enthalpy change for the net rxn (rxn=REACTION) is the sum of the enthalpy’s of the individual rxns. An indirect method of measuring enthalpy given a series of rxns. Rules for manipulating the equations: If coefficients are multiplied or divided, ΔH must be also If an equation is reversed, so is the sign on ΔH Hess’s Law Problems 1. The brown haze of smog over large cities is often due to the production of NO2 from N2 and O2. Given the information below, what is the net enthalpy change for this reaction? N2 + O2 2NO ΔH= +181 kJ 2NO + O2 2NO2 ΔH= - 113kJ If reactants and products are on proper sides of arrows, add equations together. N2 + O2 + 2NO + O2 2NO + 2NO2 N2 + 2O2 2NO2 ΔH = +118 + -113 = +68kJ More Practice 2. Calculate ΔH of S + O2 SO2 From the following enthalpy changes: 2SO2 2S + O2 2SO3 ΔH = –196kJ + 3O2 2SO3 ΔH = -760kJ 3. Calculate the ΔH of PCl3 + Cl2 PCl5 From the following enthalpy changes 2P + 3Cl2 2PCl3 delta H = -640kJ 2P + 5Cl2 2PCl5 delta H = -760kJ One More 4. Calculate the delta H prime for 2S + 2OF2 SO2 + SF4 From the following rxns: OF2 + H2O O2 + 2HF SF4 + 2H2O SO2 + 4HF S + O2 SO2 delta H = -277kJ delta H = -828kJ delta H = -297kJ 12-4 Calorimetry Calorimetry- the study of heat flow and heat measurement. These experiments determine the heats (enthalpy changes) of rxns by measuring the changes of temperature in a device called a calorimeter (we’ll make one in lab…) Heat Capacity-the amount of heat needed to raise the temperature of the object by 1 degree Celsius. This is dependent on the mass and the composition of the substance. Heating lots of water vs. a drop… Heating water vs. metal… SPECIFIC HEAT Specific The heat- amount of heat needed to raise the temperature of 1g of water by 1 Co. Water’s specific heat is 4.184 J TRUE OR FALSE? A small temperature change means that a small quantity of heat was transferred…. FALSE! A small change in temperature may be produced by a large quantity of heat… …in an object that has a very large heat capacity! HEAT & TEMP ARE RELATED, BUT NOT THE SAME! Specific heat is the amount of heat needed to raise the temperature The Equation qsur = m x C x (Tf-Ti) & qrxn = - qsur q = heat transfer measurement made in a calorimeter. Sur = surrounding solution Rxn = overall reaction m = mass of water C = specific heat (Tf-Ti) is final temperature minus initial temperature Sample Problem 1. 4.25 g NH4NO3 dissolves in 60.0 g of water and the temp drops from 21.0 oC to 16.9oC. Calculate . NH4NO3 NH4 NO3 qsur = m x C x (Tf-Ti) qsur = (60.0g)(4.184J/goC)(16.9-21.0oC) = -1.03 x 103 J (heat transferred to the surroundings) qrxn = 1.03 x 103 J (heat transferred in the reaction) But this is not yet… Everything Goes Through Moles! 4.25g NH4NO3 1mol NH4NO3 1.03 x103 J 80.0gNH4NO3 1 mol NH4NO3 Is this reaction endo or exo thermic???? Again! 13.7g of solid lead II nitrate dissolves in 85.0g of water in a calorimeter, the temp drops from 23.4C to 19.7 C. Calculate the delta H for this rxn Fill in the correct numbers and solve Convert the lead II nitrate grams to mols Convert the mols from above to J for 1 mol (or the given balanced eq.) Do problem #6 on page 397 text It’s a good one… 12-5 What is Heat? The Caloric Theoryscientists like Lavoisier of the 1700's believed heat was fluid. That it flows… Count Rumford and James Joule did experiments indicating that heat was a form of energy. The Kinetic Theory -In the 1900 century scientists began to understand that heat was the result of motion and vibration of particles of matter. Heat-the transfer of kinetic energy from a hotter object to a cooler one. Now, hopefully you understand why temperature is a measure of ___________ ____________! Entropy – randomness in a reaction with regard to energy that cannot be reharnessed (heat!) ΔS Disorder A reaction is ALWAYS spontaneous if ΔS + and ΔH – A reaction is NEVER spontaneous if ΔS - and ΔH + If both signs are the same, spontaneity depends on temperature