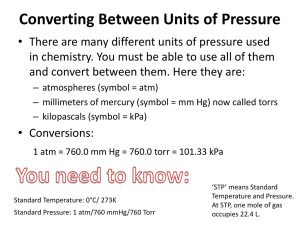
One of the main assumptions of the kinetic molecular theory of
advertisement

PHASES OF MATTER AND KINETIC MOLECULAR THEORY One of the main assumptions of the kinetic molecular theory of gases is that the particles of an ideal gas — A B C D must be single atoms instead of molecules are in constant motion must be maintained at very high pressures must be highly chemically reactive An ice-skating rink has tubes under its floor to freeze the water. Salt water is cooled well below the freezing point of water and pumped through the tubes to freeze the water in the rink. Why can the salt water be cooled so low without freezing? A B C D Salt has a very low freezing point. Adding salt to water lowers its freezing point. Movement of the salt water through the tubes keeps it in the liquid state. The salt water is constantly absorbing energy from its surroundings The decrease in the vapor pressure of the solvent that occurs when a solute is added to the solvent causes an increase in the boiling point and decrease in the melting point of the solution. What probably causes water to have the highest specific heat of the substances listed above? A Molecule size B Molecular mass C Strong hydrogen bonds D High density of ice Soda water is a solution of carbon dioxide in water. This solution is composed of a — A gaseous solute in a gaseous solvent B liquid solute in a liquid solvent C gaseous solute in a liquid solvent D liquid solute in a gaseous solvent The average kinetic energy of a sample of water molecules is — A increased as the temperature is decreased B increased as the temperature is increased C unaffected by temperature changes D always equal to zero If the heat of fusion of water is 80 cal/g, the amount of heat energy required to change 15.0 grams of ice at 0º C to 15.0 grams of water at 0º C is— A 80 cal B 560 cal C 1200 cal D 2400 cal 80cal 15g H x g A gas cylinder is filled with 4.00 moles of oxygen gas at 300.0 K. The piston is compressed to yield a pressure of 400.0 kPa. What is the volume inside the cylinder? A 3.19 dm3 B 6.25 dm3 C 24.9 dm3 D 31.5 dm3 A gas cylinder is filled with 4.00 moles of oxygen gas at 300.0 K. The piston is compressed to yield a pressure of 400.0 kPa. What is the volume inside the cylinder? nRT PV nRT V P kPa dm3 4.00m olx8.31 x300K m ol V 400.0kPa A gas cylinder is filled with 4.00 moles of oxygen gas at 300.0 K. The piston is compressed to yield a pressure of 400.0 kPa. What is the volume inside the cylinder? A 3.19 dm3 B 6.25 dm3 C 24.9 dm3 D 31.5 dm3 If the heat of fusion is 32.2 kJ/mol, the amount of heat energy required to melt 5.67 grams of FeO is — A B C D 2.54 kJ 3.26 kJ 5.32 kJ 18.3 Kj If the heat of fusion is 32.2 kJ/mol, the amount of heat energy required to melt 5.67 grams of FeO is — Molar Mass of FeO is 1(55.8) + 1(16.0) = 71.8 g 5.67gFeO 1m olFeO x 0.0790m olFeO 71.8 g 32.2KJ 0.0790m olFeO x 2.54KJ m ol If the heat of fusion is 32.2 kJ/mol, the amount of heat energy required to melt 5.67 grams of FeO is — A B C D 2.54 kJ 3.26 kJ 5.32 kJ 18.3 Kj Water has several unique properties such as high boiling point, high surface tension, and low vapor pressure. The type of attraction that best accounts for these unique properties is — A dispersion forces B coordinate covalent bonding C hydrogen bonding D ionic bonding An experiment yielded the above temperature and time information. What is the freezing point of the material in this experiment if the material is a solid at time zero? A -25º C B 0º C C 25º C D 50º C A sample of hydrogen gas is collected over water at 25º C. The vapor pressure of water at 25º C is 23.8 mmHg. If the total pressure is 523.8 mmHg, what is the partial pressure of the hydrogen? Dalton’s Law P1 + P2 +… = PT Phydrogen + Pwater = P Total A 23.8 mmHg B 47.6 mmHg C 500.0 mmHg D 523.8 mmHg 523.8 mm Hg - 23.8 mm Hg 500.0 mm Hg Water molecules have the greatest kinetic energy in — A ice at 0º C B water at 100 373 0KC C water at 98º C D steam at 150º C The table above lists the melting and boiling points of some metals. Which metal remains liquid over the widest range of temperature? A Copper B Iron C Lead D Platinum According to the graph, what happens at the triple point of water? A Only ice and liquid water exist in equilibrium. B Water exists only as a solid. C Water exists only as a gas. D Ice, water vapor, and liquid water exist in equilibrium A gas cylinder with a volume of 3.00 dm3 contains 8.00 moles of oxygen gas at a temperature of 50.0 K. What is the pressure inside the cylinder? A 504 kPa B 1110 kPa C 2220 kPa D 3320 kPa PV = nRT kPa dm3 8.00m olx8.31 x50.0 K nRT m ol K P P 3 V 3.00dm Water can be made to boil above its normal boiling point of 100º C by — A decreasing the air pressure B increasing the air pressure C increasing the heat being applied D decreasing the volume of the container E. PASS THE SOL! Which numbered process represents condensation? A1 B2 C3 D4 A tank contains N2 at 1.0 atm and O2 at 2.0 atm. Helium is added to this tank until the total pressure is 6.0 atm. What is the partial pressure of the helium? A 4.0 atm B 3.0 atm C 2.0 atm D 1.0 atm 6.0 atm – (1.0 atm + 2.0 atm) = If the heat of fusion of water is 3.4 x 102 J/g, the amount of heat energy required to change 15.0 grams of ice at 0º C to 15.0 grams of water at 0º C is — A 3.4 x 102 J B 2.4 x 103 J C 5.1 x 103 J D 1.0 x 104 J If the heat of fusion of water is 3.4 x 102 J/g, the amount of heat energy required to change 15.0 grams of ice at 0º C to 15.0 grams of water at 0º C is — 2 3.4 x10 J x15g g If the heat of fusion of water is 3.4 x 102 J/g, the amount of heat energy required to change 15.0 grams of ice at 0º C to 15.0 grams of water at 0º C is — A 3.4 x 102 J B 2.4 x 103 J C 5.1 x 103 J D 1.0 x 104 J If the pressure exerted on a confined gas is doubled, then the volume of the gas — A increases four times B decreases by one-fourth C is doubled D is halved Which of the following liquids would exhibit the highest vapor pressure at 25.0º C? A Water, boiling point = 100º C B Glycerine, boiling point = 290º C C Ethyl alcohol, boiling point = 78.3º C D Ether, boiling point = 34.6º C Equal quantities of different liquids are placed in closed manometers at 20º C. Which liquid has the highest vapor pressure? A sample of oxygen gas is collected over water at 22º C and 98.67 kPa pressure. If the partial pressure of the water is 2.67 kPa, the partial pressure of the oxygen is — A 93.33 kPa B 96.00 kPa C 98.66 kPa D 101.33 kPa A sample of oxygen gas is collected over water at 22º C and 98.67 kPa pressure. If the partial pressure of the water is 2.67 kPa, the partial pressure of the oxygen is — P1 P2 P3..... Pn Ptotal Poxygen Pwater Ptotal P P P 98total .67 kPa water 2.67 kPA Oxygen 96.00kPa A sample of oxygen gas is collected over water at 22º C and 98.67 kPa pressure. If the partial pressure of the water is 2.67 kPa, the partial pressure of the oxygen is — A 93.33 kPa B 96.00 kPa C 98.66 kPa D 101.33 kPa The specific heat capacity of a substance is the quantity of heat required to change the temperature of 1 gram of a substance by — A 1º C B 5º C C 10º C D 20º C Which of the following could cause a gaseous substance to liquefy? We can liquefy a gas by either cooling it and/or pressurizing it. A. An increase in pressure B. An increase in volume C. An increase in temperature D. A decrease in number of moles According to Charles’ law, the volume of a fixed amount of gas is directly proportional to — Simply stated, Charles' Law indicates that for a fixed amount of gas (fixed number of moles) at a fixed pressure, the volume is proportional to the temperature. A. isoelectric mixture B. vapor concentration C. barometric pressure D. Kelvin temperature V1 V2 T1 T2 The energy required to melt a solid into a liquid is called — A. heat of vaporization B. heat of fusion C. cooling curve D. triple point Industrial deep-sea divers must breathe a mixture of helium and oxygen to prevent a disorienting condition known as nitrogen narcosis. If a diver’s tank is filled with a helium-oxygen mixture to a pressure of 170 atmospheres and the partial pressure of helium is 110 atmospheres, the partial pressure of the oxygen is — P1 + P2 + P3 + … = PTotal A. 60 atm B. 110 atm C. 140 atm D. 280 atm Therefore Ptotal – PHe = PO 170 atm -110 atm = 60 atm A sample of nitrogen occupies 10.0 liters at 25oC and 98.7 kPa. What would be the volume at 20oC and 102.7 kPa? A. 7.87 L B. 9.45 L C. 10.2 L D. 10.6 L P1V1 P2V2 T1 T2 Use combined gas law and convert degrees Celcius to Kelvin. Each balloon was filled with an identical number of moles of gas. Which of the following best explains why balloon B is larger than balloon A? A. The gas in balloon A is under less pressure. B. The gas in balloon A is warmer. C. The gas in balloon B is under more pressure. D. The gas in balloon B is warmer. Line D represents water. If the atmospheric pressure in a flask is lowered to 70 kPa, water would boil at what temperature? A. 32° C B. 70° C C. 92° C D. 100° C Solid magnesium has a specific heat of 1.01 J/g °C. How much heat is given off by a 20.0 gram sample of magnesium when it cools from 70.0° C to 50.0° C? A. 202 J B. 404 J C. 808 J D. 1010 J H C p xmxT 1.01J o H o x 20.0 gx20 C g C Which of the following best describes sublimation? A. A solid melting to a liquid B. A solid melting to a liquid, which then evaporates C. The movement of gaseous particles so that they fill the space they occupy D. A solid forming a gas The freezing point and the boiling point of water can be altered by a variety of techniques. Which of the following has little or no effect on the boiling point of water? A. Increasing the air pressure above the liquid B. Adding alcohol to the water C. Adding sodium chloride to the water D. Increasing the amount of water Standard atmospheric pressure is 101.3 kPa. According to the graph, which of these four liquids boils at the lowest temperature? A. 1 B. 2 C. 3 D. 4 The higher the vapor pressure the lower the boiling point! Between points 2 and 3, energy is being used to — A. melt ice B. heat water C. evaporate water D. heat water vapor According to Boyle’s law, the relationship between the pressure and volume of a gas at constant temperature is — A. numerically equivalent B. inversely proportional C. positively correlated D. totally unrelated Charles’ Law states that if a given quantity of gas is held at a constant pressure, then its volume is directly proportional to the absolute temperature. This law explains why — A. the pressure of a gas increases when volume decreases B. a gas-filled balloon expands when it is heated C. solids require heat in order to change into gases D. some gases only react with each other at high temperatures The amount of energy needed to raise one gram of a substance one degree Celsius is a characteristic property known as — A. heat of formation B. heat of vaporization C. molar heat of fusion D. specific heat capacity The total pressure of an O2-Ar-He gas mixture is 755 mm Hg. If the partial pressure of Ar is 174 mm Hg and the partial pressure of He is 389 mm Hg, then the partial pressure of O2 is — A. 192 mm Hg B. 282 mm Hg C. 366 mm Hg D. 563 mm Hg 755 mm Hg – [174 mmHg + 389 mm Hg] = 192 mm Hg Study the SOL Test is Next!