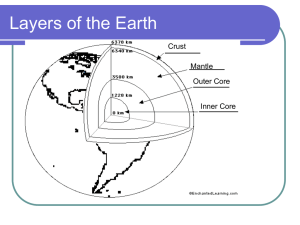
Volcano Intro ppt
advertisement

What is a volcano? A hill with a crater? Does magma need to be involved? Does it matter? Lecture material about Introduction to Volcanology, covering, Heat in the earth, where magma comes from and how, earth’s mantle, tectonics and convection, basalt and why it is fundamental, where volcanoes are. Thanks to Wendy Bohrson and Glen Mattioli who provided many of the slides. Magma Plumbing System Melts form in mantle Pool in magma chambers Magma eventually erupts Volcanology Study of generation of magma, transport of magma, and shallow-level or surface processes that result from intrusion and eruption of magma Volcanology Physical and chemical behavior of magmas Transport and eruption of magma Formation of volcanic deposits What do we need for volcanism? Thermal energy Material to melt Ability to erupt Earth’s Energy Budget • Solar radiation: 50,000 times greater than all other energy sources; primarily affects the atmosphere and oceans, but can cause changes in the solid earth through momentum transfer from the outer fluid envelope to the interior • Radioactive decay: 238U, 235U, 232Th, 40K, and 87Rb all have t1/2 that >109 years and thus continue to produce significant heat in the interior; this may equal 50 to 100% of the total heat production for the Earth. Extinct short-lived radioactive elements such as 26Al were important during the very early Earth. • Tidal Heating: Earth-Sun-Moon interaction; much smaller than radioactive decay • Primordial Heat: Also known as accretionary heat; conversion of kinetic energy of accumulating planetismals to heat. • Core Formation: Initial heating from short-lived radioisotopes and accretionary heat caused widespread interior melting (Magma Ocean) and additional heat was released when Fe sank toward the center and formed the core What are the sources of heat within Earth? Primordial/accretional energy Radioactive decay “Natural” Radioactivity • Elements (determined by Z) typically exist as a mix of isotopes which have different atomic weights (eg 39K and 40K, where Z=19). • Isotopes may be stable, radioactive or radiogenic. • 39K is stable, 40K is radioactive, 40A and 40Ca radiogenic. • Decay of radioactive isotopes has a very predictable rate: N = Noe-t . • This decay occurs spontaneously everywhere and is not influenced by changes in T, P or composition! • Decay reactions of many types occur: 40K-> 40Ca + electron + heat. • Discovered by Marie Curie. Natural Radioactivity is exploited by volcanologists and petrologists. 1. Radiometric dating. System of 40K->40A leads to K/A and A/A dating methodology. These use the age eqn and depend on purging of A at time of eruption. 2. Radioactive Tracing. Use isotopic ratios of elements to tell where the magma came from. Ex: 87Sr/86Sr this is radiogenic/stable, so it can measure the amounts of radioactive parent= 87Rb Rates of Heat Production and Half-lives Radioactive Decay The Law of Radioactive Decay dN N dt dN or = N dt time D = Net - N = N(et -1) age of a sample (t) if we know: D the amount of the daughter nuclide produced N the amount of the original parent nuclide remaining the decay constant for the system in question The K-Ar System 40K either 40Ca or 40Ar – 40Ca is common. Cannot distinguish radiogenic 40Ca from non-radiogenic 40Ca – 40Ar is an inert gas which can be trapped in many solid phases as it forms in them The appropriate decay equation is: 40Ar = 40Ar o+ e 40K(e-t -1) Where e = 0.581 x 10-10 a-1 (electron capture) and = 5.543 x 10-10 a-1 (whole process) • Blocking temperatures for various minerals differ • 40Ar-39Ar technique grew from this discovery Heat Production through Earth History Earth Structure How do we know the composition of the mantle? Peridotite bodies (e.g., ophiolites) Xenoliths Cosmochemical Evidence/Meteorites Ophiolites Seismic velocity is plotted on the horizontal axis versus depth below the seafloor on the vertical axis. The different seismic layers are marked on the plot with geologic interpretations of the rock units. The layers are defined by velocities and velocity gradients. Cross section through a typical ophiolite sequence is shown to the right. http://www.womenoceanographers.org/doc/KGillis/Lesson/gillis_lesson.htm Ophiolites Picture of a hillside in Cyprus. The vertical slabs of rock are dikes intruding into lavas that erupted on the seafloor. This section represents the transition from lavas to sheeted dikes and is thought to correspond to seismic Layer 2B as seen in Figure 5. Taken from the RIDGE field school in Cyprus. http://www.womenoceanographers.org/doc/KGillis/Lesson/gillis_lesson.htm Mantle Xenoliths http://www.nhm.ac.uk/mineralogy/petrology/MantleXenoliths.htm Carbonaceous Chondrites Left to right: fragments of the Allende, Yukon, and Murchison meteorites http://www.daviddarling.info/encyclopedia/C/carbchon.html Mantle vs Model CC Composition of the Mantle What is the mineralogy of the mantle? Olivine +clinopyroxene + orthopyroxene ± plagioclase, garnet, spinel (Al bearing minerals) Mineralogy of Mantle crust obvious from space that Earth has two fundamentally different physiographic features: oceans (71%) and continents (29%) from: http://www.personal.umich.edu/~vdpluijm/gs205.html global topography Differentiation of the Earth Rb>Sr Nd>Sm La>Lu Continental Crust La Lu Rb>Sr Nd>Sm La>Lu Mantle (After partial melt extraction) Rb<Sr Nd<Sm La<Lu La Lu • Melts extracted from the mantle rise to the crust, carrying with them their “enrichment” in incompatible elements – Continental crust becomes “incompatible element enriched” – Mantle becomes “incompatible element depleted” From: http://www.geo.cornell.edu/geology/classes/geo302 Radioactivity in earth materials Rock Type 238U 235U 232Th 40 ppm ppm K ppm Cont 3.9 crust Ocean .79 crust Mantle .01 0.03 18 3.5 96 .006 3 .96 18 7x10-5 0.06 1.2x10-3 0.26 Meteor .01 -ites 7x10-5 0.38 0.1 ppm Heat mWkg-1 x 10-8 0.50 Heat production decreases with depth from crust to mantle. Approximate Pressure (GPa=10 kbar) Earth’s Geothermal Gradient Average Heat Flux is 0.09 watt/meter2 Geothermal gradient = DT/ Dz 20-30C/km in orogenic belts; Cannot remain constant w/depth At 200 km would be 4000°C ~7°C/km in trenches Viscosity, which measures resistance to flow, of mantle rocks is 1018 times tar at 24°C ! Earth Interior Pressures P = rVg/A = rgz, if we integrate from the surface to some depth z and take positive downward we get DP/Dz = rg Rock densities range from 2.7 (crust) to 3.3 g/cm3 (mantle) 270 bar/km for the crust and 330 bar/km for the mantle At the base of the crust, say at 30 km depth, the lithostatic pressure would be 8100 bars = 8.1 kbar = 0.81 GPa Gravity, Pressure, and the Geobaric Gradient • Geobaric gradient defined similarly to geothermal gradient: DP/Dz; in the interior this is related to the overburden of the overlying rocks and is referred to as lithostatic pressure gradient. • SI unit of pressure is the pascal, Pa and 1 bar (~1 atmosphere) = 105 Pa Pressure = Force / Area and Force = mass * acceleration P = F/A = (m*g)/A and r (density) = mass/volume Heat Flow on Earth An increment of heat, Dq, transferred into a body produces a Proportional incremental rise in temperature, DT, given by Dq = Cp * DT where Cp is called the molar heat capacity of J/mol-degree at constant pressure; similar to specific heat, which is based on mass (J/g-degree). 1 calorie = 4.184 J and is equivalent to the energy necessary to raise 1 gram of of water 1 degree centigrade. Specific heat of water is 1 cal/g°C, where rocks are ~0.3 cal/g°C. Heat Transfer Mechanisms • Radiation: involves emission of EM energy from the surface of hot body into the transparent cooler surroundings. Not important in cool rocks, but increasingly important at T’s >1200°C • Advection: involves flow of a liquid through openings in a rock whose T is different from the fluid (mass flux). Important near Earth’s surface due to fractured nature of crust. • Conduction: transfer of kinetic energy by atomic vibration. Cannot occur in a vacuum. For a given volume, heat is conducted away faster if the enclosing surface area is larger. • Convection: movement of material having contrasting T’s from one place to another. T differences give rise to density differences. In a gravitational field, higher density (generally colder) materials sink. Magmatic Examples of Heat Transfer Thermal Gradient = DT between adjacent hotter and cooler masses Heat Flux = rate at which heat is conducted over time from a unit surface area Thermal Conductivity = K; rocks have very low values and thus deep heat has been retained! Heat Flux = Thermal Conductivity * DT Types of Thermal Energy Transfer Models of Earth’s interior converge on core Ts of 4000°C ± 500 °C Thermal energy moves from hot to cold--> thus, modes of energy transport within Earth: • Conduction • Convection • Radiation Earth Structure How do we know that convection is important? Thought experiment: Distance heat transported by conduction = sqrt (thermal diffusivity * age of Earth) • Thermal diffusivity = 10-6 m2/s • 3.2 x 107 sec/yr How do we know that convection is important? 10-6 m2/s * 4.5 x 109 yr * 3.2 x 107 sec/yr = 380 km Radius of Earth = 6371 km Conclusion: barely any heat transported by conduction. Requires a convective mechanism. Convection Examples Rayleigh-Bernard Convection Convection in the Mantle from: http://www.geo.lsa.umich.edu/~crlb/COURSES/270 models convection in the mantle observed heat flow warmer: near ridges colder: over cratons from: http://www-personal.umich.edu/~vdpluijm/gs205.html examples from western Pacific blue is high velocity (fast) …interpreted as slab note continuity of blue slab to depths on order of 670 km from: http://www.pmel.noaa.gov/vents/coax/coax.html Earth’s Plates Where Volcanoes Occur Volcano geography 1. Divergent margins 2. Convergent margins 3. Intraplate 4. Hotspots Plate tectonics and magma composition 1. Divergent margins: Plate separation and decompression melting -> low volatile abundance, low SiO2 (~50%), low viscosity basaltic magmas (e.g. Krafla, Iceland) 2. Convergent margins : Mixtures of basalt from the mantle, remelted continental crust and material from the subducted slab. High volatile abundance, intermediate SiO2 (60-70%), high viscosity andesites and dacites (e.g. Montserrat, West Indies) 3. Intraplate `Hot-spot` settings: A. Oceanic: Mantle plumes melt thin oceanic crust producing low viscosity basaltic magmas (e.g. Kilauea, Hawaii) B. Continental: Mantle plumes melt thicker, silicic continental crust producing highly silicic (>70% SiO2) rhyolites (e.g. Yellowstone, USA) What are the plate tectonic settings in which magmatism occurs? Processes of Partial Melting Precursor to all igneous rocks is magma or melt (liquid rock) How does melting occur? Processes of Partial Melting Let’s first look at a phase diagram (P-T) diagram of mantle Processes of Partial Melting A simpler phase diagram (P-T) diagram of mantle Processes of Partial Melting What causes partial melting in the mantle? Two processes: Lowering of solidus by volatile addition Adiabatic Decompression Processes of Partial Melting Lowering solidus by volatile addition Temperature Processes of Partial Melting Pressure Adiabatic Decompression The Mantle Why is melting in the mantle important? Because most of the melts that make extrusive rocks on Earth originate in the mantle Approximate Pressure (GPa=10 kbar) Earth’s Geothermal Gradient Average Heat Flux is 0.09 watt/meter2 Geothermal gradient = DT/ Dz 20-30C/km in orogenic belts; Cannot remain constant w/depth At 200 km would be 4000°C ~7°C/km in trenches Viscosity, which measures resistance to flow, of mantle rocks is 1018 times tar at 24°C ! Mechanisms of melt formation 1. MOR = Adiabatic decompression Intraplate = adiabatic decompression Convergent = change in solidus by volatile fluxing Divergent settings: The Midocean Ridge Bathymetry of the East Pacific Rise Magma Chamber Structure beneath East Pacific Rise Volcanic layer transitions into sheeted dike zone, which represents feeder zone from magma chamber. Below is a sill-like magma body (1-2 km depth) that transitions to crystal mush (partially solidified zone >50% crystals). Transitional zone is solidified but hot gabbro. MORB Genesis Intraplate settings: Mantle Plumes Proposed Hot Spot Traces Magma Plumbing System for Hawaii Zone of partial melting at depth (>100 km) Magma ascends through conduit system Presence of summit reservoir and rift zones Shallow Magma Plumbing System Geometry of Magma Reservoir beneath Kilauea Convergent settings: Subduction Zone Magmatism Characteristics of Subduction Zone Magmatism Down-going, hydrated slab undergoes metamorphism and dehydration Fluids infiltrate overlying mantle “wedge” Reduces solidus and melting can occur Produces arc magmatism Relative Volumes What are the relative volumes of eruption and intrusion? What are the relative volumes of eruption and intrusion? Volumes of Igneous Rocks on Earth Convergent Margin Magma Genesis Classification of Igneous Rocks Figure 2-4. A chemical classification of volcanics based on total alkalis vs. silica. After Le Bas et al. (1986) J. Petrol., 27, 745-750. Oxford University Press. Basalt Types-Major Element Variation Alkaline and Subalkaline Rock Suites 15,164 samples Irregular solid line defines the boundary between Ne-norm rocks Le Bas et al., 1992; Le Roex et al., 1990; Cole, 1982; Hildreth & Moorbath, 1988 K2O content of subalkaline rocks K2O content may broadly correlate with crustal thickness. Low-K 12 km Med-K 35 km High-K 45 km Ewart, 1982 Yoder & Tilley Basalt Tetrahedron Yoder & Tilley, 1962; Le Maitre Terrestrial Basalt Generation Summary • MORBs are derived from the partial melting of a previously depleted upper mantle under largely anhydrous conditions at relatively shallow depths. • True primary mantle melts are rare, although the most primitive alkali basalts are thought to represent the best samples of direct mantle melts. • The trace element and isotopic ratio differences among N-MORB (normal), E-MORB (enriched), IAB, and OIB indicate that the Earth’s upper mantle has long-lived and physically distinct source regions. • Ancient komatiites (>2.5 Ga) indicate that the Earth’s upper mantle was hotter in the Archean, but already depleted of continental crustal components. Lunar Surface Apollo 15 Basalt Sample Vesicles Probably derived from CO degassing Lunar Olivine Basalt Thinsection Fe-Ti oxides Plagioclase Olivine + aligned MIs Pyroxenes Plane Polarized Light Sample collected from the SE end of Mare Procellarum by the Apollo 12 mission. Interpreted as a Lava Lake basalt. Cross Polarized Light From: http://www.union.edu/PUBLIC/GEODEPT/COURSES/petrology/moon_rocks/12005.htm Lunar Anorthosite Thinsection Pyroxenes Fractured Plagioclase Feldspar Rock is 98% fsp, An95 to An97 Plane Polarized Light Highly brecciated lunar anorthosite was collected by the Apollo 16 mission to the lunar highlands SW of Mare Tranquillitatis. It has been dated at 4.44 Ga. Cross Polarized Light From: http://www.union.edu/PUBLIC/GEODEPT/COURSES/petrology/moon_rocks/12005.htm Earth Mars-sized Impact Model for Lunar Origin Impact + 0.5 hr Impact + 5hr From: Kipp & Melosh, 1986 (above) and W. Hartmann paintings of Cameron, Benz, & Melosh models (right) Features of the Giant Impact Hypothesis • Original idea paper by Hartmann & Davis, 1975; additional geochemical research by Michael Drake and computer models by Jay Melosh and colleagues. • Impact occurs soon after Earth’s core formation event because of the small lunar Fe core and difference in bulk density (rMoon = 3.3 g/cc << rEarth = 5.5 g/cc). • Impact event must occur before formation of the lunar highlands at 4.4 Ga, which formed as a result of the crystallization of the lunar magma ocean. Lunar differentiation continues w/ basalt genesis (3.95 to 3.15 Ga). • Oxygen isotope compositions of lunar and terrestrial rocks are similar, but different from Mars and meteorites. EarthMoon must be made of the same stuff. • Volatiles are depleted in the proto-moon during impact event. This is consistent with geochemistry and petrology of lunar samples. Lunar Interior Composition From: BVSP, 1986 and Taylor, 1987 1984 Mauna Loa Eruption Phase 1: Pu’u O’o Curtain of lava Phase 1: Pu’u O’o Fire Fountain Pu’u O’o Vent with pahoehoe flows Pahoehoe flow, Kilauea Tree Molds, ~1983 Halemaumau, Kilauea Surtsey, Iceland A new volcanic island formed in 1966 Cerro Negro, Nicaragua Stromboli Volcano, Italy Paricutin, Mexico 1943-1954 Mt. Augustine, Alaska Augustine Note hummocky topography from debris avalanche, 1883 Eruption of Mt. Augustine, 1986 Crater Lake Crater Lake Ol Doinyo Lengai A sodium carbonatite volcano in the Rift Valley of East Africa Ol Doinyo Lengai A sodium carbonatite volcano in the Rift Valley of East Africa Olympus Mons, Mars A giant Martian volcano 25 km high and 700 km wide. The Island of Maui in Hawaii would fit inside the huge caldera of Olympus Mons. Sources • http://www.doubledeckerpress.com/archive.ht m • http://pubs.usgs.gov/gip/volc/types.html • http://hvo.wr.usgs.gov/ • http://cvo.wr.usgs.gov/