fluid flow /pressure in stationary fluids
advertisement

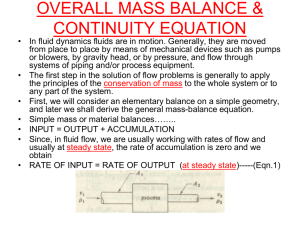
FLUID FLOW /PRESSURE IN STATIONARY FLUIDS Energy Forms of energy • Any fluid in motion possesses 4 kinds of energy Form of Energy 1. Potential or head 2. Kinetic or velocity 3. Static or Pressure 4. Heat Example - water at top of tank - water doing work on water wheel - Pressure exerted inside line - Heat generated in line due to friction Examples of conversion of energy Pumps - mechanical to velocity; mechanical to pressure Steam siphon - Pressure to velocity Water hammer - Velocity to pressure Filling tank - Pressure to velocity to potential Total Energy • The total energy that a fluid in motion has at any time is equal to the sum of the form listed above. Because energy can neither be created or destroyed, it is therefore possible to convert one form of energy to another, such as velocity into pressure. Fluid Flow Bernoulli’s Principle • when a fluid is in motion, its pressure increases whenever its velocity decreases. • Likewise its pressure decreases whenever its velocity increases Fluid • A fluid is a substance which undergoes continuous deformation when subjected to a shear stress. Factors determining Fluid Flow Viscosity • Property of fluid to resists any force that tends to produce flow. This resistance to flow [or movement] is known as viscosity • It affects the performance of pumps and the pressure drop when pumping through a given pipe line. Factors determining Fluid Flow Pipe size • For a given through put, the pressure drop increases rapidly with decreasing pipe diameter Roughness • The degree of roughness of the internal wall of the pipe affects fluid flow. Old rusty or pitted pipe would offer more resistance to flow (higher pressure drop) Pipe restrictions • The number of fittings, valves, etc affects friction loss. Type of Flow Streamline flow – fluid flows in smooth layers. Flow is in straight unbroken lines Re < 2000 Turbulent flow – there is an irregular random motion of fluid particles Re > 4000 Transition – between above. Reynolds Number Nature of flow in pipe depends on pipe diameter, the density and viscosity of the flowing fluid, and the velocity of flow. Re = dvp u Where; Re - Reynolds number d - internal diameter of pipe v - kinematic viscosity p - weight density of fluid u - dynamic viscosity The term kinematics refers to the quantitative description of fluid motion or deformation. Kinematic viscosity v = u/p u = viscosity p = density Pumping • In plant operations, we quite often want the flow to be from a low to a high pressure area. This requires use of pumps or compressors. • In the operation of pumps, pressure drop is of utmost importance. To operate a centrifugal pump, for example, the suction line can be and often is more important than discharge line. Although the discharge line may be designed so that the pump is capable of putting the required amount of liquid through it, Pumping • If the suction line is not large enough or is improperly installed, pumping difficulties will be experience. • If pressure drop is such that liquid starts to vaporize when it reaches the pump, or if suction conditions are not correct, then pump will not fill up properly and will become ‘gas bound’. • The pumping rate and discharge pressure will fall off, and pump will begin to cavitate and heat up. Flow of Gases • Gases are compressible [ liquids are non-compressible] • In moving a gas through a line, the higher the pressure on the system, the more pounds of gas that can be transferred for the same volume rate of flow. The effects on plant operations due to compressibility of a gas are: • • • • Increased pressure means reduced equipment size Gas such as air, under pressure can be used to operate pneumatic tools Hydrocarbon gases can be compressed, cooled and liquefied Refrigeration Fluid Performing Work A column of water will exert a pressure of 0.433 psi for each foot of weight. • Suppose we build a dam and raised the height of the water to 200 feet. That would mean that the pressure at the bottom of the dam would be 0.433 x 200 or 86 psi. This says that if turbines were installed at the bottom of the dam we would have a driving force of 86 psi to turn the turbines which can be used to generate electricity. Siphon • The siphon is a bent tube with branches of unequal length open at both ends and it is used to move a liquid from a higher point to a lower point, over an intermediate point higher than either. C 1 ft 2 ft A B Siphon • Since the upward force at A is greater than B, the flow will be from A to B. Suppose the tube is filled with water and placed in the vessels as shown in the figure above. The water in leg CA exerts a head or downward pressure of 1 foot of water or 0.433 psi. The water in branch CB exerts a head of 3 x 0.433 or 1.299 psi. Therefore, the net downward pressure causing flow from A to elevation B is 0.866 psi. Fluid Rates Measurement • When pressure is converted to velocity, pressure is reduced. The difference in pressure between 2 points in the line of flow is the principle on which common flow meters operate. • Orifice plate • Pitot tube • Venturi tube Pressure in Stationary Fluids Force - means a push or pull Weight - means heaviness Pressure - The push or pull or weight or weight per unit area of the surface acted upon. Pressure = __ Force_____ Area Acted upon = __ Weight____ Area Acted Upon Water weight 62.4 pounds per cubic ft, 1 foot high water would therefore exert a pressure of: 62.4lbs ft3 x 1 ft = 144 0.433 lbs ins2 Pressure Produced by Liquids • Since all fluids have weight, they create a pressure against the walls that hold them. • The pressure exerted by a liquid at any given point or location in a vessel depends upon the height of liquid above it. • This pressure is independent of the shape of the vessel • The pressure on the btm of all vessels below will be the same Pressure Produced by Liquid (con’t) • Different liquids weigh different amount for the same volume and therefore would create different pressures. 1 cubic foot of water weighs 62.4 pounds. If this cubic foot of water were put in a box 1 foot high, 1 foot wide, and 1 foot long, the total force on the bottom of the box would be 62.4 pounds. This total force would be distributed over an area one foot square or 144 square inches. The pressure on a given square inch would be 62.4 pounds divided by 144 square inches [0.433 psi]. • Pressure Produced by Liquid (con’t) • If a box were filled with water that was 2 feet high and held 2 cubic feet the pressure would equal 62.4 x 2 /144 or 0.866 psi. • Each foot of water height will develop 0.433 psi. For example a 100 foot tower, if filled with water, would create 43.3 psi at the bottom of the tower. Density • Weight of one cubic foot of material is called the density of that material Pressures Produced By Gases • The deep layer of air which blankets the earth exerts a pressure much like the water pressure at the bottom of the ocean. This pressure is known as atmospheric pressure and is about 14.7 PSI at sea level. At higher elevation, the atmospheric pressure falls. • The weight of air causes an atmospheric pressure of 14.7 psia. Less than 14.7 psia is a vacuum. Gauge Pressure • Most of pressure gauges are calibrated so that “0” on the gauge is atmospheric pressure. • Reading therefore is called PSIG Standard Pressure • The volume of a given sample of a gas has almost no meaning unless the pressure is specified. • For uniformity, 760 mm of mercury, or 1 atm, has been adopted as the standard pressure. • All specific properties of gases such as density – are always at standard pressure. Vacuum • When a pressure is less than atmospheric pressure, it is called a vacuum. A vacuum is a lack of air or fluid. Laws Governing Gas Under Pressure Boyle Law • At constant temperature, the volume of a fixed weight of a given gas is inversely proportional to the pressure under which it is measured. P x V = K (at constant temp) P1V1 = P2V2 at constant temperature If the pressure is doubled, volume is halved If the pressure is halves, volume is doubled Charles’ Law • At constant pressure, the volume of a fixed weight of gas is directly proportional to the absolute temperature V = kT (at constant pressure) V1 = T1 (at constant pressure) V2 T2 Dalton’s Law of Partial Pressure • Each gas in a gaseous mixture exerts a partial pressure equal to the pressure which it would exert if it were the only gas present in the same volume, and the total pressure of the mixture is the sum of the partial pressures of all the component gases. P total = P1 + P2 +P3 ……. Raoult’s Law Ideal solution - exhibits no change in the properties of its constituents beyond that of dilution. - For our present purpose an ideal solution is most usefully defined in terms of generalization known as Raoult’s law. Raoult’s Law - At any given temperature, the vapor pressure of any component of a solution (i.e, its partial pressure in the mixed vapor in equilibrium with the solution) is equal to the product of the mole fraction of that component in the solution and its vapor pressure in thepure liquid state at the same temperature. Raoult’s Law Raoult’s Law (con’t) Thus in a solution composed of 2 volatile liquid A and B, the vapor pressure of which in the pure state are PAo and PBo respectively, we may write: PA = XAPAo PB = XBPBo Total vapor pressure P of an ideal solution: P = XAPAo + XBPBo Problem solving 1. A sample of gas occupies a volume of 86.8 ml at a pressure of 730 mm of mercury and a temperature of 27oC. What will be its volume at standard pressure and 27oC? Solution: Standard pressure = 760 mm hg V1 = 86.8 ml P1 = 730 mm V2 = ? Ml P2 = 760 mm Pressure is increased from 730 to 760 mm, the volume is therefore decreased, and the original volume must be multiplied by a fraction less than 1. V2 = 86.8 x 730/760 = 83.4 ml Problem Solving 2. Calculate the volume that 22.4 liters of a gas measured at the standard pressure would occupy at a pressure of 732 mm hg. V1 = 22.4 liters V2 = ? Liters P1 = 760 mm P2 = 732 mm Since the pressure is decreased to 730/760 of its original value, the volume is increased by the factor 760/732 V2 = 22.4 liters x 760/732 = 23.3 liters Problem Solving 3. A gas measures 83.4 ml at 1 atm pressure and a temperature of 27 oC. Calculate its volume at standard condition. V1 = 83.4 ml V2 = ? Ml T1 = 27 + 273 = 300 oK T2 = 273 oK Since the temperature is decreased, the volume will also be decreased. The original volume must therefore br multiplied by a fraction less than 1, namely 273/300: V2 = 83.4 ml x 273/300 = 75.9 ml Problem Solving 4. A sample of gas occupies a volume of 86.8 ml at a pressure of 730 mm and a temperature of 27 oC. What will be its volume at standard condition? V1 = 86.8 ml V2 = ? Ml T1 = 300 oK T2 = 273 oK P1 = 730 P2 = 760 Pressure is increased from 730 to 760 mm, volume should decrease, we therefore multiply the original volume by 730/760. Temperature decrease from 300 to 273 oK, likewise volume decreases, hence we multiply by the temperature fraction 273/300, therefore: V2 = 86.8 ml x 730/760 x 273/300 = 75.9 ml. Problem Solving 5. A certain gas measures 546 ml at a pressure of 1 atm and a temperature of -80 oC. Calculate the volume it would occupy at a pressure of 1.5 atm and a temperature of 30 oC. First we change the centigrade temperature to absolute: - 80 oC = 80 + 273 = 193 oK 30 oC = 30 + 273 = 303 oK V1 = 546 ml V2 = ? ml P1 = 1 atm P2 = 1.5 atm T1 = 193 oK T2 = 303 oK V2 = 546 ml x 1/1.5 x 303/193 = 571 ml Joule-Thomson Effect • Gases are cooled upon sudden expansion from high to low pressure, even if they do no external work. • As gas expands the molecules pull apart from each other, and work must be done to overcome the cohesive forces that tend to hold the molecules together. Since this work is done at the expense of the kinetic energy of the molecules of the gas, the temperature is lowered on expansion. Standard Volume • Volume measured at standard condition ie temp = 60 oF • Pressure = 1 atm (14.7psi) • At standard condition one (1) mole of gas occupies 22.4 liters