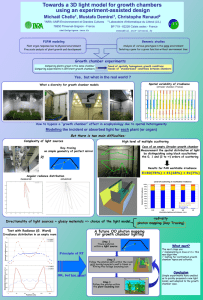
THE GAS LAWS
advertisement
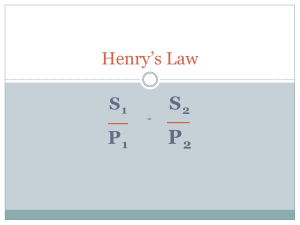
STEM THE SCIENCE OF THE HYPERBARIC CHAMBER Brief history of the Hyperbaric Chamber in the Cayman Islands After fundraising and generous donations by Cayman businesses, a reconditioned hyperbaric chamber was bought in the U.S. and placed in a hut behind Cayman Clinic in 1972. It was run by the British Sub-aqua Club. It is now in a room at the Hospital near the Forensic Science lab and the Morgue. THE HYPERBARIC CHAMBER A few years ago THE HYPERBARIC CHAMBER From Right THE HYPERBARIC CHAMBER From Left THE HYPERBARIC CHAMBER THE HYPERBARIC CHAMBER Looking Inside THE HYPERBARIC CHAMBER Inside looking Out Dive Physics THE GAS LAWS Most Important to the Hyperbaric Chamber Boyle’s Law Dalton’s Law of Partial Pressure Henry’s Law THE GAS LAWS - Pressure Pressure = force per unit area The Gas Laws refer to absolute pressure (Pabs). We are subject to a pressure at the surface because of the weight of 10 miles of air above us. The pressure at the surface is l atmosphere (l atm). A pressure gauge is made to read zero at the surface despite the weight of the atmosphere, and therefore reads pressure extra to the already existing atmospheric pressure. Each 33ft, or 10 meters of water, exert the same pressure as atmospheric air at sea level. (Continued on next slide) 10 THE GAS LAWS - Pressure continued Absolute pressure refers to Gauge pressure +1 atm. ie the total pressure including atmospheric Example the gauge pressure 99 ft below the surface of the sea is 3 atm due to the weight of the water. However the absolute pressure is 4 atm, due to the combined effect of the water plus the weight of the atmosphere pressing down on the surface of the water. Pabs = Pg+ 1 atm 11 THE GAS LAWS – Pressure Compressor Room and Pressure Tanks THE GAS LAWS – Pressure Gauge on Chamber Pressure Units 1 atmosphere (atm) at sea level is (approximately) = 1 kg per square cm (1 kg/cm2) (1 bar) = 14.7 pounds per square inch (p.s.i.) = 760mm of mercury (torr) = 29.92in Hg = 101,300 (Newtons per square meter) (pascals) (Pa) = 33ft of sea water Boyles Law: -The volume of a fixed mass of gas at a constant temperature varies inversely as the (absolute) pressure. P x V = Constant (As the pressure goes up the volume goes down & vice-versa.) P1 x Vl = P2 x V2 If a diver fills his lungs and then holds his breath while he ascends, the volume of his lungs will expand until either he decides to breathe out or the lungs rupture (embolism). 15 Depth Pressure Gas Volume 0’ 1 atm Full 33’ 2 atm ½ full 66’ 3 atm 1/ 3 full 99’ 4 atm ¼ full 132’ 5 atm 1/ 5 full 16 QUESTION A diver ascends from 132 ft to 99ft while holding his breath. If his lungs contained 4 litres of air at 132ft, what is the volume at 99ft? P2xV2 = P1xV1 (Boyles Law) V2= P1xV1 / P2 = 5atm ab x 4/ 4atm abs= 5 litres A trainee diver at 33ft shoots to the surface while holding his breath. If his lungs held 4 litres at 33 ft what is the volume at the surface. P2xV2=P1xV1 V2=P1xV1 / P2 = 2atm x 4 / 1atm = 8 litres. Both divers ascended 33ft, which one is most likely to suffer an embolism? DALTONS LAW. Dalton’s Law is a concept that states that the total pressure exerted by a mixture of gases is the sum of the partial pressures that each gas would exert if it alone filled the container. Air at the surface contains 21% oxygen and 79% nitrogen (Including the 1% of other gases with the nitrogen). air at the surface(1atm) = ppO2 0.21 atm +ppN2 0.79 atm air at 33 fsw(2atm) = ppO2 0.42 atm + ppN2 l.58 atm The importance of Daltons Law to divers and the Chamber is that the beneficial/toxic effect of gases is dependent on their partial pressures, not the total pressure of the gas mixture that contains them. 18 HENRY’S LAW Henry’s Law of solubility states that the amount of gas absorbed by a particular liquid is proportional to the partial pressure of the gas . E.g. If you triple the absolute pressure then the amount of gas absorbed by the liquid will be tripled. ppN in air at a pressure of 66ft of seawater = 3 x .79=2.37 atm Therefore the divers blood would eventually contain three times the amount of nitrogen at sixty six feet than it does at the surface. 19 HYPERBARIC OXYGEN TREATMENTS Dive related Arterial Gas Embolism Decompression Sickness Other Treatments Wound Healing Restoration of Circulation (Advanced Diabetes) Skin Grafts Carbon Monoxide Poisoning HYPERBARIC OXYGEN TREATMENTS 1. Arterial Gas Embolism Caused by over-expansion of alveolar tissue resulting from gas trapped in the lung expanding on ascent and subsequent entry of gas bubbles into the bloodstream. HYPERBARIC OXYGEN TREATMENTS 2. Decompression Sickness Caused by rapid reduction of environmental pressure resulting in Nitrogen (previously dissolved in the body tissues) being released as bubbles in the blood stream. TYPES OF DECOMPRESSION SICKNESS Type I DCS Joint pain and skin bends only. Caused by the physical effects of he bubbles and immune reaction on the tissues. TYPES OF DECOMPRESSION SICKNESS Type II DCS Typically due to bubbles in the nerve tissue of the spinal cord or brain, causing a wide range of neurological problems. TYPES OF DECOMPRESSION SICKNESS The Chokes Nitrogen bubble froth in the blood in right side of heart and/or in the lungs. The heart compresses the bubbles rather than pumping the blood. Bubbles in the alveoli capillaries prevent gas exchange and result in extreme shortness of breath. BENEFITS OF HIGH ppO2 Normally people at sea level breathe oxygen at a partial pressure of 0.21atm In the hyperbaric chamber at 60ft the patient is breathing pure oxygen at a pressure of almost 3 atm Next 2 slides – places where higher than normal ppO2 is available To Combat Pressure reduction in Aircraft at Altitude Passengers breathe pure oxygen in order to remain conscious 27 To Reduce Chance of Decompression Sickness Divers can breathe nitrox, a mixture of 32% Oxygen and 68% Nitrogen 28 HYPERBARIC OXYGEN Pure Oxygen is used as well as pressure in hyperbaric treatments. 1. It reduces the amount of nitrogen taken into the lungs during breathing to zero, thus speeding up the release of dissolved nitrogen from the blood into the lungs. HYPERBARIC OXYGEN 2. The high partial pressure of oxygen in the lungs means there is far more oxygen dissolved in the blood plasma than usual. Despite the patient’s reduced circulation this allows more oxygen to reach the tissues and so promotes healing and reduces swelling. OXYGEN CYLINDERS OXYGEN MASKS PROBLEMS WITH HIGH ppO2 1. Breathing a high ppO2 for an extended period can lead to Oxygen Toxicity – this affects the brain and causes a convulsion similar to an epilectic fit. In the chamber the patient is on pure oxygen for 20 minute periods followed by a 5 minute air break. Next slide – Table 6 – Decompression Sickness showing air breaks CLOCKS – INNER LOCK CLOCKS – OUTER LOCK OTHER CYLINDERS PROBLEMS WITH HIGH ppO2 2. The high ppO2 in the chamber is a serious fire hazard. Oxygen sometimes leaks from patients mask, increasing % O2 in Inner Lock Flammable materials, including paper, avoided. No equipment or material that could cause a spark is allowed inside. The air supplied to chamber is very dry so increases the risk of sparks from static electricity. Chamber has “fire wands” and a high pressure sprinkler system. There is an oxygen analyzer on the exhaust air, set to alarm when the oxygen level reaches 24%. Next slides – fire wands. Oxygen analyzer FIRE WAND OXYGEN ANALYZER BANNED ITEMS LIGHTS ARE EXTERNAL 2 QUESTIONS? THE END INTERESTED IN BEING ON THE CHAMBER TEAM? Call John Elliot 9161198 Or Ann Elliott 9161957 To find out when the next training course will be offered Diving physics questions 1) A gauge reads a pressure of 3 atmospheres. What is the actual pressure? …………………… 2) Why must you never hold your breath as you ascend in the chamber? ______________________ 3) A balloon is blown up in the chamber. What will happen to the size of this balloon when you ascend? _________________________________________ 4) What will happen to the volume of the liquid in a drinking water bottle during ascent? 47 The General Gas Law A combination of Boyles Law and Charles Law leads to the General Gas Law: P1 xVl = P2xV2 = a constant T1 T2 If you heat a fixed volume of gas the pressure will go up. If you leave a dive tank already filled to a high pressure out in the sun, then the pressure will rise until... If you reduce the volume, and try to keep the temperature constant, the pressure will go up. WHENEVER YOU USE THE GAS,LAWS YOU MUST USE ABSOLUTE PRESSURE AND KELVIN DEGREES (otherwise it won’t work!)