Document
advertisement

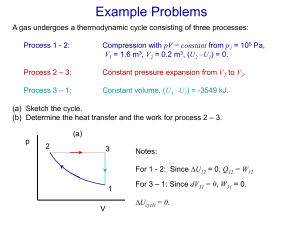
Chapter 17 The First Law of Thermodynamics Thermodynamic Concepts • Thermodynamic system: able to exchange heat with its surroundings • State variables: p, V, T, ... describe the thermodynamic system • Thermodynamic process: changes the state ( p, V, T, ...) of the system Thermodynamic Process Heat Q: can leave or enter system Work W: • system can do work on its surroundings • surroundings can do work on the system thermodynamic system: can exchange heat with its surroundings state of system: (p, V, T, ...) thermodynamic process: changes state of the system thermodynamic process: changes state of the system We’ll focus on the roles of: • Heat Q • Work W Heat Q: can leave or enter system Q > 0: heat added to system Q < 0: heat removed from system Sign Conventions for Q Q > 0: heat added to system Q < 0: heat removed from system • Consistent with sign of DT from earlier: Q = mc DT or Q = nC DT Work W: W > 0: system does work on its surroundings W < 0: surroundings does work on the system Sign Conventions for W W > 0: system does work on surroundings W < 0: surroundings does work on system • (the ‘opposite perspective’ as in mechanics) (a) Q > 0, W = 0 (b) Q < 0, W = 0 (c) Q = 0, W > 0 (d) Q = 0, W < 0 (e) Q > 0, W > 0 (f) Q < 0, W < 0 Work done when volume changes dW Fdx ( pA)dx p( Adx) pdV Work done when volume changes dW pdV 2 W pdV 1 Work done when volume changes dW pdV 2 W pdV 1 Work W is path-dependent dW pdV 2 W pdV 1 • W = area under graph of the function p(V) • W depends on initial and final states (1, 2) • W depends on path taken (intermediate states) Q (= heat transferred) is also path-dependent Thermodynamic Concepts • Thermodynamic system: described by state variables (p, V, T, ..) • Thermodynamic process: changes the state ( p, V, T, ...) of the system • Heat Q, Work W: ‘path-dependent’: values depend on process Heat Q and Work W • Q and W are not properties of the system (Q enters or leaves the system) (W is done on or by the system) • We can measure the difference: Q – W • Q – W is related to a property of the system Q–W • We choose a thermodynamic system • We take the system between a fixed initial final state for many different processes • For each process, we measure Q – W • Experiment surprises us! Q–W • For this setup, we always find: • Q – W has same value for all processes • Q – W depends only on initial, final state • Q – W is path-independent (these are three equivalent statements) Q–W Since Q – W depends only on state variables: Q – W = a change in a property of the system We define U = ‘internal energy’ of system: Q – W = DU First Law of Thermodynamics Q – W = DU or Q = W + DU • Generalizes conservation of energy from just mechanical energy to include heat energy First Law of Thermodynamics Q – W = DU or Q = W + DU • The heat energy Q added to a system goes into work W and change in internal energy U First Law of Thermodynamics Q – W = DU or Q = W + DU • (Notation: U is not simply ‘potential energy’) Laws of Thermodynamics Zeroth Law: ‘every thermodynamic system has a property called temperature T’ First Law: DU = Q – W ‘every thermodynamic system has a property called internal energy U’ DU = Q – W Recall: • Q can be > 0, < 0, = 0 • W can be > 0, < 0, = 0 Thus: • DU can be > 0, < 0, = 0 Free Expansion • Break partition • Let gas expand freely into vacuum Free Expansion • gas is in equilibrium at initial and final states • gas is not in equilibrium between initial and final states Free Expansion • Set-up for process: Q = 0 (insulation) W = 0 (no pushing) • First Law says: DU = Q – W = 0 Free Expansion • For the gas: Dp , DV are nonzero • Experiment shows: • low density (‘ideal’) gases have DT = 0 between initial and final states Free Expansion • For the gas: Dp , DV are nonzero • Experiment: DT = 0 • First Law: DU = 0 • Conclude: For an ideal gas, U only depends on T Laws of Thermodynamics Zeroth Law: ‘every thermodynamic system has a property called temperature T’ First Law: DU = Q – W ‘every thermodynamic system has a property called internal energy U’ First Law of Thermodynamics Q – W = DU or Q = W + DU • Generalizes conservation of energy: • Heat energy Q added to a system goes into both work W and change in internal energy U Thermodynamic Processes Process Free Expansion: Cyclic: Definition Q=0 W=0 closed loop Consequence DU = 0 DU = 0 Q=0+W Thermodynamic Processes Process Definition Consequence Isobaric p = constant W = p DV Isochoric V = constant W=0 Q = DU + 0 Thermodynamic Processes Process Definition Consequence Isothermal T = constant (must be slow) DU = 0 Adiabatic Q=0 (insulated or fast) 0 = DU + W Molar Heat Capacity Revisited Q = n C DT • Q = energy needed to heat/cool n moles by DT • CV = molar heat capacity at constant volume • Cp = molar heat capacity at constant pressure CV for Ideal Gases, Revisited Molecular Theory: (Ktot)av = (f/2) nRT CV = (f/2)R Monatomic: f = 3 Diatomic: f = 3, 5, 7 New language: U = (f/2) nRT CV = (f/2)R Monatomic: f = 3 Diatomic: f = 3, 5, 7 Cp for Ideal Gases • We expect: Cp > CV • Example: gas does work expanding against atmosphere • We can show: Cp = CV + R Derive this result Cp for Ideal Gases C p CV R CV R CV CV Cp • monatomic gas: CV = (3/2)R (5 / 2) R 5 1.67 (3 / 2) R 3 • diatomic gas: at low T, CV = (5/2)R (7 / 2) R 7 1.40 (5 / 2) R 5 Adiabatic Process (Q = 0) • An adiabatic process for an ideal gas obeys: TV -1 = constant value pV = another constant Derive these results Adiabatic Process (Q = 0) For an ideal gas undergoing an adiabatic process: W nCV (T1 - T2 ) CV ( p1V1 - p2V2 ) R 1 ( p1V1 - p2V2 ) -1 Derive these results Derive some isobaric results Do Problem 17-42