Thermodynamics
advertisement
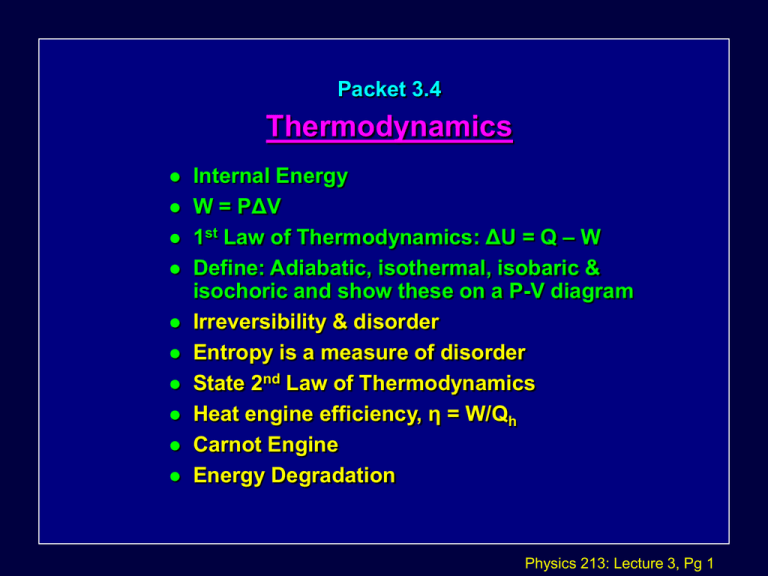
Packet 3.4 Thermodynamics Internal Energy W = PΔV 1st Law of Thermodynamics: ΔU = Q – W Define: Adiabatic, isothermal, isobaric & isochoric and show these on a P-V diagram Irreversibility & disorder Entropy is a measure of disorder State 2nd Law of Thermodynamics Heat engine efficiency, η = W/Qh Carnot Engine Energy Degradation Physics 213: Lecture 3, Pg 1 Internal Energy Is the total potential and kinetic energy of the molecules in a substance. Potential energy is associated with intermolecular forces. Kinetic energy includes both translational and rotational motion. When we consider an ideal gas, the intermolecular forces are assumed to be zero! Internal energy of a gas comes only from the random kinetic energy of the atom of the gas. Physics 213: Lecture 3, Pg 2 Ideal Gas & Internal Energy Ek = 1/2mv2 = 3/2kT U =3/2nRT Δ U = 3/2 nR Δ T so, U = 3/2 NkT Physics 213: Lecture 3, Pg 3 Ideal Gas & Internal Energy What is the internal energy of 30 moles of oxygen gas at room temperature? U =3/2nRT Physics 213: Lecture 3, Pg 4 Ideal Gas & Internal Energy If the room were moving at a high velocity would that mean the internal energy of the gas would be greater? Physics 213: Lecture 3, Pg 5 Work done on or by a gas Imagine compressing a gas by exerting a force on the piston from the outside. Consider heating the piston and it expands to perform work. W = F x D & F = PA W = P (A x D) W=PΔV Physics 213: Lecture 3, Pg 6 Example A gas is compressed at constant pressure 2.00 x 105 Pa from a volume of 2.00 m3 to a volume of 0.500 m3. What is the work done on the gas. If the temperature initially was 40˚ C what is the final temperature of the gas? Physics 213: Lecture 3, Pg 7 Thermodynamic Processes CLICK HERE – TO GO OVER EACH PROCESS!! Isochoric Const. Volume Isobaric Const. Pressure Isothermal Const. Temp Remember! Area under curve is Work Done! Physics 213: Lecture 3, Pg 8 Adiabatic Expansion Rapid expansion or compression of a gas. No Heat (Q) can flow in or out of the system. ΔQ = 0 Any work done equals a direct change in internal energy. ΔU = ΔW Bottle Rockets Diesel Engines Physics 213: Lecture 3, Pg 9 Figure 18-11 Adiabatic Heating Physics 213: Lecture 3, Pg 10 The First Law of Thermodynamics (FLT) -- Heat and work are forms of energy transfer and energy is conserved. U = Q - Wby change in total internal energy State Function heat added to system work done by the system Process Functions or U = Q + Won Physics 213: Lecture 3, Pg 11 "Process Problems" For which process is W the largest? smallest? For which process is Q the largest? smallest? Physics 213: Lecture 3, Pg 12 Physics Joke Once all the scientists die and go to heaven. They decide to play hide-n-seek and Einstein goes first. Einstein counts up to 100 and then start searching. Everyone starts hiding except Newton. Newton just draws a square of 1 meter and stands in it, right in front of Einstein. Einsteins counting ....97,98,99,100, opens his eyes and finds Newton standing in front. Einstein says "Newtons out, Newtons out." Newton denies and says I am not out. He claims that he is not Newton. All the scientists come out and he proves that he is not Newton. How?? Physics 213: Lecture 3, Pg 13 His proof: Newton says: I am standing in a square of area 1m square.. That means I am Newton per meter square.. Hence I am Pascal. Since newton per meter square = Pascal Physics 213: Lecture 3, Pg 14 Conceptual Checkpoint 18-2 Which is the adiabatic curve? Physics 213: Lecture 3, Pg 15 The second law of thermodynamics When objects of different temperatures are brought into thermal contact, the spontaneous flow of heat that results is always from the high temperature object to the low temperature object. Physics 213: Lecture 3, Pg 16 Heat Engines Energy goes in Useful Work taken out Some gets wasted Max Efficiency: TH – Tc/ Th= x 100 Physics 213: Lecture 3, Pg 17 The 2nd Law of Thermodynamics The second law of thermodynamics deals with the limitations imposed on heat engines: that is on devices whose aim is to covert heat (disordered energy) into mechanical energy (ordered energy). Physics 213: Lecture 3, Pg 18 The 2nd Law of Thermodynamics The Entropy of an isolated system never decreases. It is impossible for heat to (spontaneously) flow from a cold to a hot object. It is impossible for a heat engine working in a cycle to absorb heat and perform an equal amount of work. The most efficient heat engine operating between two given temperatures is the Carnot Engine. Physics 213: Lecture 3, Pg 19 Entropy Entropy like Internal energy is a State Function! Entropy Is a measure of the disorder of a system. ΔS = ΔQ/T If ΔQ > 0 entropy increases If ΔQ < 0 entropy decreases. Physics 213: Lecture 3, Pg 20 Philosophy Three Laws of Thermodynamics (paraphrased): First Law: You can't get anything without working for it. Second Law: The most you can accomplish by work is to break even. Third Law: You can't break even. Physics 213: Lecture 3, Pg 21