110811 - Williams Research Group
advertisement
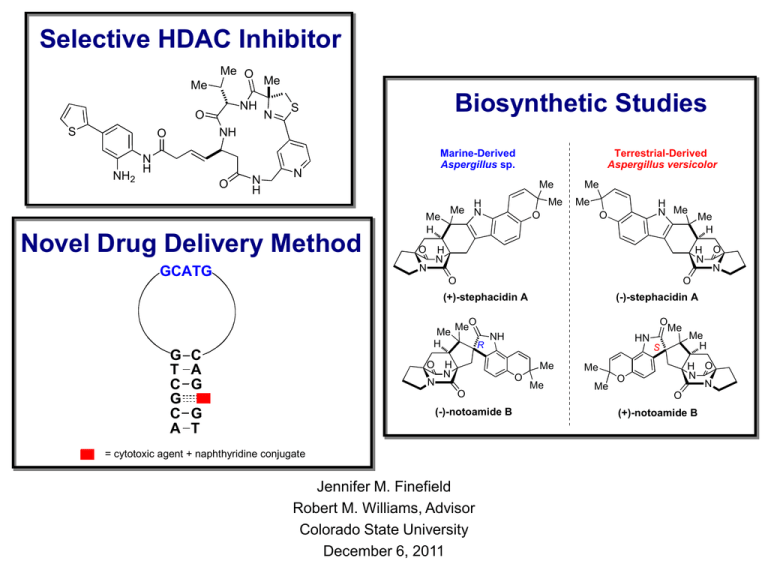
Selective HDAC Inhibitor Me O Me NH O S N Biosynthetic Studies S NH O NH2 Me N H O N H Marine-Derived Aspergillus sp. N Terrestrial-Derived Aspergillus versicolor Me Novel Drug Delivery Method O N GCATG Me H Me N Me H Me Me O H O N N H N O O (+)-stephacidin A Me H G T C G C A H N Me Me H O C A G MeO R O Me NH O H N N HN S Me O O G T (-)-stephacidin A (-)-notoamide B = cytotoxic agent + naphthyridine conjugate Jennifer M. Finefield Robert M. Williams, Advisor Colorado State University December 6, 2011 Me H O N N Me Me Me H O O (+)-notoamide B Efforts Toward the Synthesis of a Selective Histone Deacetylase Inhibitor N S Me O Me Me N NH NH H N O O O NH NH2 S DNA Packaging • For DNA to fit within the nucleus, it must be condensed • DNA is packaged into chromatin • To begin packaging, DNA is wound around histones www.med.unc.edu www.christopher_vidal.com Gene Expression: Dynamic Wrapping and Unwrapping of DNA • Histone Acetyltransferase (HAT) readies DNA for transcription • Histone Deacetylase (HDAC) returns DNA to the inactive state X • HDAC inhibitors prevent removal of acetyl residues O RO O NHR HAT RO NHR X HDAC HDAC NH3 www.med.unc.edu www.christopher_vidal.com HN O Transcriptional Control • HDAC inhibitors mimic the natural substrate • Deacetylation is prevented, eventually leading to cell death Zn-Dependent Histone Deacetylase Enzymes Q u ic k T im e ™ a n d a T I F F ( Un c o m p r e s s e d ) d e c o m p r e s s o r a r e n e e d e d t o s e e t h is p ic t u r e . Figure 2. Schematic domain structure of Class I, IIA, IIB and IV Histone Deacetylases. Deacetylase domains are depicted in color against a silver background illustrating the relative length of each individual enzyme. • HDAC enzymes are divided into different classes • Within each class, there are different isoforms • Many known HDAC inhibitors display very little selectivity for class or isoform Marks, P. A., et al., Advances in Cancer Research, 2005, 137 HDAC Inhibitors and Enhancing Selectivity O H N NHOH O HDAC1 IC50(nM) 30 HDAC3 IC50(nM) 100 vorinostat (SAHA) Surface Recognition Linker Ph HNO OR Metal Binding NH O O H RN H N NHOH N H O O vorinostat (SAHA)H2N Surface Recognition Linker Internal Cavity O 2+Zn O HN 2+ZnN H NH O Internal Cavity Internal Metal Binding Cavity • Many known HDAC inhibitors display very little selectivity for class or isoform Wiest, O. et al., J. Med. Chem. 2004, 47, 3409; Methot, J. L., et al., Bioorg. Med. Chem. Lett. 2008, 18, 973 Improving Selectivity of HDACi: Targeting HDAC1 and HDAC2 S MeO N H H N O O O NH2 N H H N MeO NH2 HDAC1 IC50(nM) 440 HDAC2 IC50(nM) 500 MeO NH2 MeO MeO MeO N H H N HDAC1 IC50(nM) 60 HDAC2 IC50(nM) 100 HDAC1 IC50(nM) 50 HDAC2 IC50(nM) 80 O S S MeO H N N H NH2 H N MeO HDAC1 IC50(nM) 50 HDAC2 IC50(nM) 70 N H OH MeO H N N H NH2 MeO MeO MeO O O O HDAC1 IC50(nM) 40 HDAC2 IC50(nM) 60 Moradei, O. M., et al., J. Med. Chem. 2007, 50, 5543 HDAC1 IC50(nM) >10,000 HDAC2 IC50(nM) >10,000 Improving Selectivity of HDACi: Targeting HDAC3 N S S O Me MeO MeO H N NO H Me Me N NH2 Me HDAC1 IC50(nM) >10,000 Increased selectivity HDAC2 IC 50(nM) >10,000 (possibly HDAC3) H N NH O Me + O Me O Improved surface O S recognition domain NH NH2 S Finefield, J. M.; Williams, R. M.; Wiest, O.; Bradner, J. Unpublished results NH O O O N O N NH NH S S largazole Improving Selectivity of HDACi: Targeting HDAC3 Ser118, present in HDAC1/2 (green) » Tyr118 in HDAC3 (blue) N S Me O N NH Me Me O H N O O NH NH2 S Zn+2 Finefield, J. M.; Williams, R. M.; Wiest, O.; Bradner, J. Unpublished results NH Key Disconnections N S Me O N NH NH Me Me H N O O O NH NH2 S Thiazoline-Pyridine Synthesis Bowers, A., et al., Org. Lett., 2009, 11, 1301 Synthesis of the Amide Isostere Bowers, A., et al., J. Am. Chem. Soc., 2009, 131, 2900 Alternate Route N S Me HO2C N 1. DCC, DMAP NHBoc MeOH, DCM N 2. TFA, DCM 52% (2 steps) Me MeO2C NH O Me HO2C OH N NH H N Me O Me O O N S HATU, HOBt i-Pr2NEt Me O Me Me N NH NH O H N Me MeO2C Me 66% N NH2 Me NH 53% S 2. TFA, DCM O THF OtBu NHBoc Me LiOH (aq.) 1. LiOH (aq.), THF MeCN Me NHBoc O S N PyBOP, i-Pr2NEt Me Me NH2 S O N N NHBoc H N O NH O 2-Thiophenyl Biaryl Synthesis S nBuLi, B(OMe)3 S Br B(OH)2 Et2O, HCl NH2 NBS NO2 HOAc NH2 Pd(PPh3)4 52% NH2 Br tri-o-tolylphosphine K2CO3, DME, H2O NO2 S 86% NO2 70% O H N HO O NO2 T3P, i-Pr2NEt DCM S MeOH:DCM 1:1 57% 71% H N Boc2O, NEt3 O NHBoc DCM S H N SnCl2 O NH2 S Final Steps and Future Direction N S N S H N Me + O NHBoc Me N NH O Me NH S Me 1. Grubbs' #2 toluene N NH O Me NH H N 2. TFA, DCM H N Me O O O O O NH NH2 S ______________________________________________________________________________________________________________________________ N S O S S O N Me N O Me NH NH O O Me Me O Thiazoline-pyridine largazole cap O Me N NH Me O N NH O O Largazole cap Me O Me N NH Me O NH O O Oxazoline-oxazole cap Design and Synthesis of a Novel Drug Delivery Method Specifically Targeted to Multiple Myeloma Cells GCATG O H3C N N N H NH O H3C N N N H O NHOH O G T C G C A C A G G T = cytotoxic agent + naphthyridine conjugate Cl N Cl NH2 Multiple Myeloma • MM is a plasma cell malignancy that can lead to bone destruction, anaemia, hypercalcaemia, and renal insufficiency • MM is associated with older age (median age 66 years) and is found to occur more often in men than women • Cause of MM remains unknown • Current treatments include a single high-dose of melphalan, velcade, and various combination treatments Trialx.com, Mahindra, A., et al., Blood Reviews 2010, 24, S5; Barlogie, B., et al., Blood 2004, 103, 20 Tumor Specific Oligonucleotide (MB8226) CDR3 gene from cell line RPMI8226 Tumor Specific mRNA Transcript UAGGCUACGUACUUAAGCG GCATG GCGAC GTCGC F Q GCATG Complementary sequence C A G C C G A G C Q G G T C G G C T C GF C Q F Quenched Probe MB8226 The Trojan Horse CGUAC GCATG GCATG ACGCTG Extra guanine (a.k.a. "G Bulge") G T C G C C A A = H3C Nakatani, K. et al., J. Am. Chem. Soc. 2000, 122, 2172 N CAG GT C A G G G T T N N H DRUG Naphthyridine Modified MM Drugs = H3C N O H N N H N DRUG O Currently undergoing clinical trials to be used H3C as N a combination N N treatment for multipleHmyeloma NHOH O vorinostat (SAHA) N-vorinostat O OH O Cl NH2 N Cl melphalan H3C N N N H Given either as a high-dose NH treatment or as partNH of a 2 O the treatment combination for of multiple myeloma Cl N Cl N-melphalan NHOH O Naphthyridine Modified Vorinostat O OMe H3PO4 + H2N N OMe NH2 H3C 89% N Coupling Conditions NH2 N O X O HO N N N H O 1. MeOH, H2SO4 OH H3C HO O 4 OMe OMe 2. KOH, MeOH O O 35% (2 steps) O HO (COCl)2, DMF OMe O Cl OMe Benzene O N H3C 80% H3C N N N H O 4 44% O NH2OH.HCl, KOH or NEt3 OMe NH2 NEt3, DMAP, DCM O O N X MeOH 0oC, rt, m.w H3C N N N H H N OH O Brown, E. V., J. Org. Chem. 1965, 30, 1607; Yoshida, M. et al., Synthesis 2008, 1099; Gediya, L. K. et al. J. Med. Chem. 2005, 48, 5047 Naphthyridine Modified Vorinostat O HO H3C (CH3CO)2O OH 98% O O Ethyl chloroformate NEt3, THF H3C N N N H O O 4 N O NH2 H3C THF O O N N N H 50% NH2OH in H2O OH OEt 27% (2 steps) O H3C N N N H N-vorinostat Mai, A. et al. OPPI Briefs 2001, 33, 391 4 54% O O N O H N O OH Naphthyridine Modified Melphalan FF Boc Boc22O O O O H H22N N 1M 1M NaOH NaOH dioxane dioxane OH OH C C66FF55OH OH O O BocHN BocHN OH OH FF FF O O FF O O EDCI, EDCI, DMF DMF BocHN BocHN H H33C C 96% 96% 92% 92% O O H H33C C N N N N H H N N O O 4M 4M HCl HCl NHBoc NHBoc CHCl CHCl33,, EtOAC EtOAC H H33C C N N N N N-Boc-melphalan N N H H NH NH22 EDCI, NMM DCM 47% 47% 21% O H 3C N N O N H H 3C N N N H NH O NH NHBoc HCl-saturated EtOAc O NH2 42% Cl N Cl Cl N N N Cl N-melphalan Nakatani, K. et al., Bioorg. Med. Chem. 2003, 11, 2347; Gullbo, J. et al., Oncol. Res. 2003, 14, 113 NH NH22 i-Pr i-Pr22NEt, NEt, DMF DMF FF 53% 53% N N Preliminary Test Results O H 3C N N N H NH O Cl N Cl N-melphalan NH2 Preliminary Test Results O H3C N N N H N-vorinostat (N-SAHA) H N O OH Studies on the Biosynthesis of Reverse Prenylated Indole Secondary Metabolites from Aspergillus versicolor and Aspergillus sp. MF297-2 Aspergillus sp. MF297-2 Aspergillus versicolor Me Me H Me N Me H O Me Me O H N Me Me H O H O N N H N N O O (+)-stephacidin A Me H O N (-)-stephacidin A MeO O Me NH HN R H N S Me O O (-)-notoamide B Me H O N N Me Me Me H O O (+)-notoamide B Reverse Prenylated Indole Secondary Metabolites 1969-2006 H Me Me N H H Me Me N H H O N N O O H N N HN Me Me O O O O H N O brevianamide A Me Me Me H O H N H O N N NH H O N N O O H H HO N N N N H O H Me N Me H O HN N H H O H O H N N brevianamide E O H N HN N Me H H Me O H O Cl Cl Me H OH Me H N H H O N N N H O Me Me O Me Me OH 3 17 N O H OMe Me O Me O NH N O H O O O Me Me Me Me N H norgeamide B H O O Me O Me Me N H Me 17 N O Me Me O N asperparaline B Me H norgeamide C O N O N O O O H N Me N O H O Me Me H Me N H N N N O N VM55596 Me Me O (+)-stephacidin A SB203105 N O O Me Me O NH O Me Me O Me Me H OH O HO NH O Me Me NH Me O O avrainvillamide (CJ-17,665) OH O Me O OH N N O paraherquamide H Me H N Me Me O Me N Me H Me O Me 20 H O Me O Me Me O O Me stephacidin B NH N 21 N Me O Me N N SB202327 O N 55 N Me O H 51 O N Me O Me Me O Me O O Me Me H H asperparaline A H Me N Me H Me H O N Me H Me N 3 2 O O O Me N Me O HO OH Me Me O Me N N N H N H Me Me H Me Me H NH Me O Me OH Me Me N H O O Me N N asperparaline C Me O Me N O paraherquamide E Me (VM54159) NH marcfortine B norgeamide A Me O Me Me H O Me N N marcfortine A Me H N Me Me NH Me H O Me O paraherquamide D MeO Me H NH paraherquamide C N Me O H N N O N H O norgeamide D Me H O Me Me O Me H N O Me O N N paraherquamide G (VM54158) O Me N Me O Me Me Me deoxy-12,13-dehydrobrevianamide E O Me Me H Me NH O Me Me O Me N N Me O O O HO OH Me N Me H O Me paraherquamide A (VM29919) marcfortine C deoxybrevianamide E O NH N NH O H N N HN Me Me O O Me Me O N N H N O Me Me malbrancheamide brevianamide F NH pre-paraherquamide brevianamide D O O Me O Me O O Me Me H paraherquamide F (VM55594) paraherquamide B VM55599 brevianamide C O Me sclerotiamide O Me O H N N Me H HO Me H NH O Me N N Me Me MeO Me H NH H O H Me Me N H H Me H O N N O N N O hydratoaustamide H Me Me N H H N O Me H O N OH O austamide brevianamide B Me HN Me Me N Me H O O H O N VM55597 O Me Me Proposed Biosynthesis of the Bicyclo[2.2.2]diazaoctane Ring System Me H H Me N Me Intramolecular H Diels-Alder O N HN O HN Me Me O N H Me H Me N O Me H H N O N H N O O (+)-stephacidin Aketo-premalbrancheamide deoxybrevianamide E Enzymatic Proposal Diels-Alder Reaction Porter and Sammes Diels-Alder (1970) Me Me Me Me O HO N O Me Me HO O N NH N N N O OH Me Me H Me Me N H N N O H O O N N O brevianamide A Porter, A. E. A. et al., Chem. Commun. 1970, 1103; Williams, R. M., Chem. Pharm. Bull. 2002, 50, 711. Me H O N Me H N O Proposed Biosynthesis of the Bicyclo[2.2.2]diazaoctane Ring System • Enzyme Controlled Stereoselectivity Me H Me Me N H Me Me H 19 19 19 H O N N H O N N O Me Me H (+)-brevianamide A O N O anti-diastereomer Me H Me N O 19 O H N N O O Me H H N O (+)-stephacidin A syn-diastereomer Paraherquamides (+)-brevianamide B Me H O N H Me N H Me Me N HO N H N O N Anti-selective O H N Me Me H [4+2] Syn-selective O O [4+2] H N Me Me OH N H Me N H N O H N Me Me H N H O N N (+)-brevianamide A Me H H O N N N O O VM55599 Reverse Prenylated Indole Secondary Metabolites 2007-2011 Me H O N MeO N OH H N Me H O Me O O N Me NH Me O Me O (-)-notoamide B O H Me Me O N H Me H N N O O OMe notoamide F O O H Me Me HO N N Me H Me O O N O N H O Me notoamide C N H N H O Me Me notoamide D OH Me H Me N Me H Me O OH O notoamide H OMe notoamide G 17 N Me MeO O O N H notoamide J HO Me Me N Me H O Br HN Me Me O O H Me N N O O NH O H N N Me N O Me Me O chrysogenamide A Me O Me Me O O N notoamide I OMe O N H O O H O N N O HN Me Me O Me Me H H O N N O (-)-versicolamide B O 3 notoamide R Me H O N H N Me N O H N 17 H O N H 3-epi-notoamide C Me OH O Me H N Me Me H (-)-stephacidin A N notoamide Q Me O O Me Me H O H N NH Cl O notoamide N Me H Me N Me H MeO H N notoamide M N N O H H notoamide P Me O Me O Me Me H N N Me OH O HN N H O Me Me notoamide L Me H N 17 3 Me Me Me O Me H N H O O N O HO N O H notoamide K HO notoamide O Me N Me H O Me Me Me H2 N O O O O Me OH O Me H N N H O H O HO H O HN H H N N 3 2 H O notoamide E Me Me HO Me N Me H 17 Me Me Me Me H Me N Me H NH O Me O (+)-notoamide B O HO N H O Me N O O Me N N O H 3 H O N N Me H O Me Me H Me H HN H N notoamide A H O Me MeO Me Me O NH Me O O (+)-versicolamide B Me Isolation of the Notoamides: New Addition to the Stephacidin Family • 2007: Aspergillus sp. MF297-2 Me H MeO N OH Me H R O H N N O O notoamide A Me O Me O Me H N N O H 3 Me N H O N H Me H H Me Me O N O Me Me H Me N Me H H N N H deoxybrevianamide E Kato, H. et al., Angew. Chem. Int. Ed. 2007, 46, 2254 Me HO O sclerotiamide O Me O N H N O (+)-stephacidin A N O Me Me notoamide D notoamide C NH N 3 2 Me Me O HO H O Me O NH O R O (-)-notoamide B H N NH O H N N Me O Me Me H MeO Me O H Isolation of the Notoamides: New Addition to the Stephacidin Family • 2008: Aspergillus versicolor NRRL 35600 Me Me O O Me H N Me Me H HN S H O N N Me H O N N Me O (-)-stephacidin A Me H Me H O O N H N N H O O HO H brevianamide F N N HN O Me Me N H O Me Me norgeamide D Greshock, T. J. et al., Angew. Chem. Int. Ed. 2008, 47, 3573 S Me O O (+)-versicolamide B H O NH H N O (+)-notoamide B Me O OH Me Antipodal Natural Products Aspergillus sp. MF297-2 Aspergillus versicolor Me O Me H Me N Me H Me Me O H N Me Me H O H O N N H N N O O (+)-stephacidin A (Aspergillus ochraceus) Syn Me H O MeO O Me NH S Me O O Me O Me R Me Me O O Me H H O N N (-)-versicolamide B H O N N Me O Me O (-)-notoamide B HN Me H HN R H N N (-)-stephacidin A (+)-notoamide B Me H O N Me O NH S H N Me O O Anti Me (+)-versicolamide B Tsukamoto, S. et al., Org. Lett. 2009, 11, 1297; Greshock, T. J. et al., Angew. Chem. Int. Ed. 2008, 47, 3573 Isolation of the Notoamides: New Addition to the Stephacidin Family • 2008-2010: Aspergillus sp. MF297-2 H O Me N O H Me Me N H O Me H N N Me Me O O O O H OMe notoamide F HO N HN O N H notoamide J H O O Me OH O Me Me H N N O Me H Me O Me MeO N O H N N OH O notoamide H OMe notoamide G Me H Me O OH O Me Me N Me H HN notoamide O N Me H O Me O Br Me Me N H H2N Me H Me N O O H Me N N O O notoamide I N N O Me O OH O NH O H N N N H O Me Me notoamide L HN MeO Me H N 17 3 Me O HO O O Me Me O H notoamide K HO N Me H N N H O H O HO O O HO Me N Me H notoamide E Me HO O Me Me H Me N Me H NH Cl O notoamide N notoamide M Me O O Me Me H N N Me H O Me N O H O Me notoamide P Me N H N OMe O notoamide Q H Me N Me H O Me HN O Me O N H N OH O Me O notoamide R Tsukamoto, S. et al: JACS, 2009, 131, 3834; JNP 2008, 71, 2064; OL 2009, 11, 1297; JNP 2010, 73, 1438 Me O Me Me Me H H O N N O (-)-versicolamide B Isolation of Notoamide E: A Potential Biosynthetic Precursor • 2008-2010: Aspergillus sp. MF297-2 Tsukamoto, S. et al., J. Am. Chem. Soc. 2009, 131, 3834 Proposed Biosynthetic Pathway: Notoamide E H OH O N N N NH O O [ox] H Me Me Me Me O Me N H N H O Me Me Me notoamide E achiral azadiene [4+2] Aspergillus sp. Me Me H O N MeO NH H N [ox] Me O O (-)-notoamide B pinacol O Me N Me HN Me Me O O Me H H O N N (-)-versicolamide B O Me Me O O [ox] pinacol Me H O N N pinacol O C6-epi-stephacidin A Greshock, T. J. et al., Angew. Chem. Int. Ed. 2008, 47, 3573 Me Me H H O N N O O O (+)-notoamide B (-)-stephacidin A + H N Me Me H HN [ox] H O N N H N + Me O Me H N Me Me H O (+)-stephacidin A Me O Me H Me N Me H Aspergillus versicolor H Me N Me H O N Me Me O H N [ox] pinacol O C6-epi-stephacidin A Me H O N Me O NH H N Me O O (+)-versicolamide B Me Synthesis of [13C]2-Notoamide E Me HO Cl 3 steps Boc2O, DMAP N H HO NO2 NCS N H BocO CH3CN DMF 75% Cl Me Me Me Me Me Me 9-BBN N H BocO NEt3, THF N H BocO 80% Me Me TFA CH2Cl2 HO N H 0.1% CuCl22H2O DBU, CH3CN 97% 56% 81% Me Me O Me Me N H Me Me 1,2-dichloro benzene 95% O Me Me N H NMe2 aq. H2CO aq. Me2NH AcOH 95% Me Me O N H Me Me Tsukamoto, S. et al., JACS 2009, 131, 3834; Grubbs, A. W. et al., TL 2005, 46, 9013; Grubbs, A. W. et al., ACIE 2007, 46, 2257 Synthesis of [13C]2-Notoamide E CO2Et Ph N Ph NMe2 Me Me N H O 1. CO2Et = 13C PBu3, CH3CN, Me Me 2. 1 M HCl, CH2Cl2 O Me Me N H NH2 Me Me O H 1. FmocCl 10% Na2CO3 dioxane NHFmoc Me Me 2. Me3SnOH O ClCH2CH2Cl Me Me 74% 76% OH N H O EtO OEt O H N H O Cl H O 86% N morpholine NHFmoc Me Me HATU, iPr2NEt CH3CN N H O H THF 88% 1:1 = cis:trans Me Me O Me Me N H N 17 12 N O H Me Me + O notoamide E Aspergillus sp. A. versicolor Tsukamoto, S. et al., J. Am. Chem. Soc. 2009, 131, 3834 O H H Me Me N H N H N O H Me Me [13C]2-Notoamide E Incorporation Study with Aspergillus sp. MF297-2 O Me Me H 12 HO N O H O O H O Me Me 12 N H O Me H N O H Me Me O H =13C N 17 12 notoamide E O Me Me • N H N H OH OH notoamide E2 O Me Me O Me Me H N 17 O H H H N O H Me Me O Me Me N H O Me Me 3-epi-notoamide C (1.26 mg) O H N O H Me Me O 12 O Me Me N Me Me N 17 N O notoamide E4 No labeled bicyclo[2.2.2]diazaoctane containing metabolites were produced Tsukamoto, S. et al., J. Am. Chem. Soc. 2009, 131, 3834 H OH OH notoamide E3 O N H N 17 12 N 17 12 N O H notoamide D notoamide C (0.33 mg) Aspergillus sp. 12 Me Me N 17 O N O N H Me H N 17 H [13C]2-Notoamide E Incorporation Study with Aspergillus versicolor H O Me Me H O N N O H Aspergillus versicolor H Me Me O Me Me N H notoamide E =13C H O Me N H Me Me Me N N O N H O O HO NH O H N O Me Me H notoamide C notoamide D 6.2% incorporation 6.0% incorporation O Me Me H N N O H O Me N H Me 3-epi-notoamide C trace amount detected Finefield, J. M.; Williams, R. M. et al., Tetrahedron Lett. 2011, 52, 1987 H O Possible Precursors Leading to Stephacidin A H O H N H N N H Me Me H O O H P450 N H HO deoxybrevianamide E O H N N H Me Me H O DMAPP N H HO NH O P450 H Me Me N H O Me Me [ox; IMDA] Me Me notoamide S notoamide E X [ox; IMDA] [ox; IMDA] Me O N H N O H Me Me Enzyme 6-hydroxydeoxybrevianamide E [ox; IMDA] N Me Me H Me N Me H O H N N O ketopremalbrancheamide P450 H Me N Me H O H N N OH Enzyme DMAPP O H Me N Me H O H N N O notoamide T Me OH Me P450 H Me N H O H N N O stephacidin A O Synthesis of Deoxybrevianamide E and 6-Hydroxydeoxybrevianamide E Kato, H.; Nakamura, Y.; Finefield, J. M.; Umaoka, H.; Nakahara, T.; Williams, R. M.; Tsukamoto, S., TL 2011, 52, 6923 Synthesis of Ketopremalbrancheamide O H OEt NH2 N H HO HATU, + HO C 2 Me Me EtO H N Boc iPr 2NEt CH3CN O O N H Me Me HO N H 99% O H N H N OH PBu3, DEAD N O H Me Me N Me H N H 78% O 2.4:1 = syn:anti N H Me N H N O ()-syn, ketopremalbrancheamide Me H + 2. 2-hydroxypyridine toluene O N O H Me Me CH2Cl2 94% 1. TFA, CH2Cl2 87% 1:1 = cis:trans O H H Boc N O N 20% aq. KOH H Me N H N O ()-anti N N MeOH N H Me Me OH Biosynthetic Breakthrough: Characterization of the ()-Notoamide Biosynthetic Gene Cluster notB orf1 notA notD notC notH notF notE notG notJ notI notL notK notN notM notO notP notR notQ Ding, Y.; de Wet, J. R.; Cavalcoli, J.; Li, S.; Greshock, T. J.; Miller, K. A.; Finefield, J. M.; Sunderhaus, J. D.; McAfoos, T. J.; Tsukamoto, S.; Williams, R. M.; Sherman, D. H., JACS 2010, 132, 12733 Biosynthetic Breakthrough: Identification of Two Prenyltransferases notB orf1 notD notA notE notC H N O H N H Me Me N H H O notH notF DMAPP N H notI notG NotF HN DMAPP H O brevianamide F HO N H notN notK notM notO notP notR notQ NotC DMAPP ketopremalbrancheamide deoxybrevianamide E O H notL N O H Me NH HMe N O H H O H Me N Me NN H O O N NotC notJ deoxybrevianamide E H Me N Me H N H O N H X Me Me NotC NotC N DMAPP DMAPP H N H X O N X = H or OH 6-hydroxydeoxybrevianamide E Me Me O H N OH NotC N H DMAPP Me O Me 6-hydroxyketo premalbrancheamide X = H or OH Ding, Y.; de Wet, J. R.; Cavalcoli, J.; Li, S.; Greshock, T. J.; Miller, K. A.; Finefield, J. M.; Sunderhaus, J. D.; McAfoos, T. J.; Tsukamoto, S.; Williams, R. M.; Sherman, D. H., JACS 2010, 132, 12733 Early Steps in the Biosynthetic Pathway N O H NotF N H Me Me DMAPP N H H O P450 NotG/H deoxybrevianamide E O H HO N H O H N N H Me Me H O 6-hydroxydeoxybrevianamide E NotC DMAPP N H HO Me Me N N H Me Me O H H O P450 N H O Me Me N notoamide C or 3-epi-notoamide C H N O H Me Me NotB + notoamide D notoamide E notoamide S X Me Me H O N H Me N H N O (+)-stephacidin A Me O Feeding Study with [13C]2-[15N]-6Hydroxydeoxybrevianamide E Aspergillus versicolor NRRL 35600 O 8.4% incorporation O H HO N H Me Me N MeN H Me H HO O Aspergillus sp. MF297-2 H N 12 18 N H H O O N H notoamide J = 13C N = 15N No incorporation into advanced metabolites Finefield, J. M.; Williams, R. M. et al., JOC 2011, 76, 5954; Finefield, J. M.; Williams, R. M.; Tsukamoto, S. et al., TL 2011, 52, 6923 Possible Enantio-diverging Pathways from Notoamide S Notoamide S Incorporation Study with Aspergillus versicolor O H O O H N H HO Me Me N N H Me Me N H N O Me Me H H N O H Me Me N H O notoamide E notoamide S N = 15N = 13C N N N H O Me O Me Me H notoamide D 6.2% 13C incorp. Aspergillus versicolor Me Me H O notoamide C 6.4% 13C incorp. H O O HO Me Me Me Me N N O H Me H O H N Me Me H (-)-stephacidin A O 6.2% 13C incorp. H O N N O Me HN Me Me O O Me H H O N N HN Me O (+)-notoamide B 6.4% 13C incorp. Unlabeled synthesis of notoamide S: McAfoos, T. J. et al., Heterocycles 2010, 82, 461 Results from feeding study: Finefield, J. M.; Tsukamoto, S.; Williams, R. M. et al., unpublished results Me O Me Me H H O N N O (+)-versicolamide B 6.5% 13C incorp. Notoamide S Incorporation Study with Aspergillus sp. MF297-2 O H N H HO O Me Me H N N H Me Me H O N O H Aspergillus sp. Me Me H O N H HO Me O Me Me H N Me N O H Me Metabolite 2 Me notoamide S N = 15N = 13C O H O Me Me Tsukamoto, S. et al., unpublished results N H H O N H HO Metabolite 1 N N H N O H Me Me notoamide E Notoamide S: Additional Biosynthetic Insight Me Me Me H Me N Me H Aspergillus sp. O H N H HO Me Me N N N H Me Me O O H H O notoamide S IMDA N H HO N N H Me Me H O Me Me Aspergilus notoamide S versicolor Me H Me H Me N O [ox] O H N N O Me (+)-notoamide T Me Aspergillus O versicolor HO Me OH Me O (+)-stephacidin A H N Me Me H HMeO N Me N H N Me Me O [ox] O H (-)-stephacidin A H O N N O (-)-notoamide T H N H N Me Me H H O N N O (-)-stephacidin A Notoamide T: Precursor Incorporation Studies Me Me Me H N Me Me H HO O Me Me A. versicolor H O N N H N Me Me H O = 13C (-)-stephacidin A O 4.7% 13C incorp. O ()-notoamide T HN H O N N Me H O N N O Me Me H O (+)-notoamide B 0.6% 13C incorp. ________________________________________________________________________________________________________________________________ Me HO Me Me H N Me Me H H O N N O ()-notoamide T Aspergillus sp. = 13C Me H O N HO Me N Me H Me O NH R H N Me O Me O O H N N O Metabolite A (-)-notoamide B Me HO Me N Me H O H N N Finefield, J. M., Tsukamoto, S.; Williams, R. M. et al., unpublished results O O Metabolite B OH Me O Me O Notoamide S: Additional Biosynthetic Insight O H N H HO Me Me N N H Me Me Me H O Aspergillus versicolor Me H N Me Me H O H O N N O (-)-stephacidin A notoamide S ? O H N H HO Me Me N N H Me Me H O O Me Aspergillus versicolor HN H O N N Me Me Me H O O (+)-notoamide B notoamide S Biosynthetic Precursor Incorporation Study Me Me H N Me Me H O H O N N O Me Aspergillus versicolor O (-)-stephacidin A =13C Me H Me N Me H O N HN Me HN + H O N N Me O Me O Me Me H Me Me O O O Me H H O OH N N O (+)-sclerotiamide 5.0% incorporation (+)-notoamide B 2.7% incorporation H N O (+)-stephacidin A ________________________________________________________________________________________________________________________________ Me H Me N Me H O N H N O ()-stephacidin A Me Me Me O Aspergillus sp. H O =13C N O NH + H N Me O O (-)-notoamide B 8.1% incorporation Finefield, J. M.; Tsukamoto, S.; Williams, R. M. et al., Org. Lett. 2011, 13, 3802 Me Me H O N O Me NH H N Me HO O O (-)-sclerotiamide 6.8% incorporation Me Characterization of the ()-Notoamide Biosynthetic Gene Cluster • ()-Notoamide Biosynthetic Gene Cluster notB orf1 notA • notD notC notH notF notE notG (+)-Notoamide Biosynthetic Gene Cluster Li, S.; Sherman, D. H. et al., unpublished results notJ notI notL notK notN notM notO notP notR notQ Current Postulated Biogenesis of the Notoamides and Stephacidins Summary Me O Me Me NH O S N H Aspergillus versicolor Me Me H Me N Me H N G T C G C A N N H O Aspergillus sp. MF297-2 O S N NH O NH2 GCATG O Me H N Me Me H O H O N N H N O H3C N N O Me NH O H N N HN S Me O O (-)-notoamide B O O H3C N N N H NH O (-)-stephacidin A MeO R NHOH N H O (+)-stephacidin A Me H G T = cytotoxic agent + naphthyridine conjugate Me O C A G Me H O N N Me Me Cl O N Me H O (+)-notoamide B Cl NH2 Acknowledgements Prof. Robert M. Williams Prof. David H. Sherman Prof. Sachiko Tsukamoto Williams Research Group Sherman Research Group Tsukamoto Research Group Dr. James Berenson







