16b
advertisement

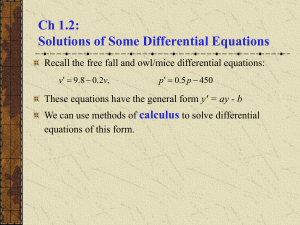
Heat Equations of Change I Outline So far… 1. Heat Transfer Mechanisms 2. Conduction Heat Transfer 3. Convection Heat Transfer 4. Combined Heat Transfer 5. Overall Shell Heat Balances 6. Heat Equations of Change Outline 6. Heat Equations of Change 7.1. Derivation of Basic Equations 7.1.1. Differential Equation for Heat Conduction 7.1.2. Energy Equation 7.1.3. Buckingham Pi Method 7.2. Unsteady-state Conduction 7.2.1. Gurney-Lurie Charts 7.2.2. Lumped Systems Analysis Differential Equation for Heat Conduction Consider a differential element balance: Assumptions: 1. Solid conduction thermal resistance only. 2. Constant density, thermal conductivity and specific heat. Differential Equation for Heat Conduction Consider a differential element balance: Rate of HEAT in - out: ∆𝑦∆𝑧 𝑞𝑥 𝑥 − ∆𝑦∆𝑧 𝑞𝑥 𝑥+∆𝑥 Rate of HEAT generation: 𝑔 ∆𝑥∆𝑦∆𝑧 Rate of HEAT accumulation: In Chem 16, this is mcPdT 𝜕𝑇 𝜌𝑐𝑝 ∆𝑥∆𝑦∆𝑧 𝜕𝑡 Differential Equation for Heat Conduction Consider a differential element balance: Heat Balance: 𝜕𝑇 ∆𝑦∆𝑧 𝑞𝑥 − ∆𝑦∆𝑧 𝑞𝑥 + 𝑔 ∆𝑥∆𝑦∆𝑧 = 𝜌𝑐𝑝 ∆𝑥∆𝑦∆𝑧 𝜕𝑡 𝑥 𝑥+∆𝑥 Dividing by ∆𝑥∆𝑦∆𝑧 : 𝑞𝑥 𝑥 − 𝑞𝑥 ∆𝑥 𝑥+∆𝑥 𝜕𝑇 + 𝑔 = 𝜌𝑐𝑝 𝜕𝑡 Taking the limit ∆𝑥 → 0: 𝜕𝑞𝑥 𝜕𝑇 − + 𝑔 = 𝜌𝑐𝑝 𝜕𝑥 𝜕𝑡 Differential Equation for Heat Conduction Consider a differential element balance: 𝜕𝑞𝑥 𝜕𝑇 − + 𝑔 = 𝜌𝑐𝑝 𝜕𝑥 𝜕𝑡 Substituting Fourier’s Law: Noting that k is constant: Extending to 3D space: 𝜕 𝜕𝑇 𝜕𝑇 − −𝑘 + 𝑔 = 𝜌𝑐𝑝 𝜕𝑥 𝜕𝑥 𝜕𝑡 𝜕2𝑇 𝜕𝑇 𝑘 + 𝑔 = 𝜌𝑐𝑝 2 𝜕𝑥 𝜕𝑡 𝑘 𝛻 2𝑇 𝜕𝑇 + 𝑔 = 𝜌𝑐𝑝 𝜕𝑡 Differential Equation for Heat Conduction Consider a differential element balance: 𝑘 Recall the definition of thermal diffusivity: 𝛻 2𝑇 𝜕𝑇 + 𝑔 = 𝜌𝑐𝑝 𝜕𝑡 Measure of how quickly a material can carry heat away from a source. 𝑘 𝛼= 𝜌𝑐𝑝 Dividing everything by k: Differential Equation for Heat Conduction 𝛻2𝑇 𝑔 1 𝜕𝑇 + = 𝑘 𝛼 𝜕𝑡 Differential Equation for Heat Conduction Differential Equation for Heat Conduction 𝛻 2𝑇 𝑔 1 𝜕𝑇 + = 𝑘 𝛼 𝜕𝑡 Simplifications of the equation: 1) No heat generation: 2) Steady-state: 3) Steady-state & no heat generation: 1 𝜕𝑇 𝛻 𝑇= 𝛼 𝜕𝑡 𝑔 2 𝛻 𝑇+ =0 𝑘 2 𝛻2𝑇 = 0 Fourier’s Second Law of Conduction Poisson’s Equation Laplace’s Equation Differential Equation for Heat Conduction Differential Equation for Heat Conduction 𝛻 2𝑇 𝑔 1 𝜕𝑇 + = 𝑘 𝛼 𝜕𝑡 The equation in different coordinate systems: 1) Rectangular: 2) Cylindrical: 3) Spherical: 𝜕 2 𝑇 𝜕 2 𝑇 𝜕 2 𝑇 𝑔 1 𝜕𝑇 + 2+ 2+ = 2 𝜕𝑥 𝜕𝑦 𝜕𝑧 𝑘 𝛼 𝜕𝑡 1 𝜕 𝜕𝑇 1 𝜕 2 𝑇 𝜕 2 𝑇 𝑔 1 𝜕𝑇 𝑟 + 2 2+ 2+ = 𝑟 𝜕𝑟 𝜕𝑟 𝑟 𝜕𝜃 𝜕𝑧 𝑘 𝛼 𝜕𝑡 1 𝜕 𝜕𝑇 1 𝜕 𝜕𝑇 1 𝜕 2 𝑇 𝑔 1 𝜕𝑇 2 𝑟 + 2 sin 𝜃 + 2 2 + = 𝑟 2 𝜕𝑟 𝜕𝑟 𝑟 sin 𝜃 𝜕𝜃 𝜕𝜃 𝑟 sin 𝜃 𝜕𝜙 2 𝑘 𝛼 𝜕𝑡 Differential Equation for Heat Conduction Example! Determine the steady-state temperature distribution and the heat flux in a slab in the region 0 ≤ x ≤ L for thermal conductivity k and a uniform heat generation in the medium at a rate of g0 when the boundary surface at x = 0 is kept at a uniform temperature T0 and the boundary surface at x = L dissipates heat by convection into an environment at a constant temperature T∞ with a heattransfer coefficient h. Differential Equation for Heat Conduction Example! Assumptions (or given): 1. Steady-state 2. Unidirectional heat flow (x only) 3. Constant k, ρ, cP, and h. Differential Equation for Heat Conduction: 𝑔 1 𝜕𝑇 𝛻 𝑇+ = 𝑘 𝛼 𝜕𝑡 2 𝑑 2 𝑇 𝑔0 + =0 2 𝑑𝑥 𝑘 Differential Equation for Heat Conduction Example! 𝑑2 𝑇 𝑔0 + =0 2 𝑑𝑥 𝑘 After 1st and 2nd integration: 𝑑𝑇 𝑔0 = − 𝑥 + 𝐶1 𝑑𝑥 𝑘 1 𝑔0 2 𝑇 𝑥 =− 𝑥 + 𝐶1 𝑥 + 𝐶2 2𝑘 Differential Equation for Heat Conduction Example! 𝑑𝑇 𝑔0 = − 𝑥 + 𝐶1 𝑑𝑥 𝑘 1 𝑔0 2 𝑇 𝑥 =− 𝑥 + 𝐶1 𝑥 + 𝐶2 2𝑘 Boundary conditions: 𝑎𝑡 𝑥 = 0, 𝑎𝑡 𝑥 = 𝐿, 𝑇(0) = 𝑇0 𝑑𝑇 𝑘 = ℎ 𝑇∞ − 𝑇(𝐿) 𝑑𝑥 *The second B.C. denotes that the heat leaving by conduction is equal to the heat entering by convection. Differential Equation for Heat Conduction Example! 𝑑𝑇 𝑔0 = − 𝑥 + 𝐶1 𝑑𝑥 𝑘 1 𝑔0 2 𝑇 𝑥 =− 𝑥 + 𝐶1 𝑥 + 𝐶2 2𝑘 Applying B.C. 1: C2 = T0 Applying B.C. 2: 𝑘 − 𝑔0 𝑔0 2 𝐿 + 𝐶1 = ℎ 𝑇∞ + 𝐿 − 𝐶1 𝐿 − 𝑇0 𝑘 2𝑘 𝐶1 ℎ𝐿 + 𝑘 = ℎ 𝑇∞ − 𝑇0 − 𝑔0 𝐿 ℎ𝐿 + 2𝑘 2𝑘 Differential Equation for Heat Conduction Example! Applying B.C. 1: C2 = T0 Applying B.C. 2: 𝑔0 𝑔0 2 𝑘 − 𝐿 + 𝐶1 = ℎ 𝑇∞ + 𝐿 − 𝐶1 𝐿 − 𝑇0 𝑘 2𝑘 𝑔0 𝐿 𝐶1 ℎ𝐿 + 𝑘 = ℎ 𝑇∞ − 𝑇0 − ℎ𝐿 + 2𝑘 2𝑘 ℎ 𝑇∞ − 𝑇0 𝑔0 𝐿 ℎ𝐿 + 2𝑘 𝐶1 = − ℎ𝐿 + 𝑘 2𝑘 ℎ𝐿 + 𝑘 𝐶2 = 𝑇0 1 𝑔0 2 𝑇 𝑥 =− 𝑥 + 𝐶1 𝑥 + 𝐶2 2𝑘 After substitution… Differential Equation for Heat Conduction Example! 1 𝑔0 2 𝑇 𝑥 =− 𝑥 + 𝐶1 𝑥 + 𝐶2 2𝑘 After substitution… 1 𝑔0 2 ℎ 𝑇∞ − 𝑇0 𝑔0 𝑥𝐿 ℎ𝐿 + 2𝑘 𝑇 𝑥 =− 𝑥 + 𝑥− + 𝑇0 2𝑘 ℎ𝐿 + 𝑘 2𝑘 ℎ𝐿 + 𝑘 Further manipulation into a desired form: 1 𝑔0 2 𝑇∞ − 𝑇0 𝑇 𝑥 − 𝑇0 = − 𝑥 + 𝑘 2𝑘 1+ ℎ𝐿 2𝑘 𝑥 𝑔0 𝑥𝐿 1 + ℎ𝐿 − 𝐿 2𝑘 1 + 𝑘 ℎ𝐿 Differential Equation for Heat Conduction Example! 1 𝑔0 2 𝑇∞ − 𝑇0 𝑇 𝑥 − 𝑇0 = − 𝑥 + 𝑘 2𝑘 1+ ℎ𝐿 2𝑘 𝑥 𝑔0 𝑥𝐿 1 + ℎ𝐿 − 𝐿 2𝑘 1 + 𝑘 ℎ𝐿 Manipulating into a desired form even further: 𝐿2 𝑇∞ − 𝑇0 𝑥 𝑔0 𝑇 𝑥 − 𝑇0 = − 𝑘 𝐿 2𝑘 1+ ℎ𝐿 2𝑘 1+ ℎ𝐿 𝑘 1+ ℎ𝐿 Now, we introduce a new dimensionless number… 𝑥 𝑥 − 𝐿 𝐿 2 Differential Equation for Heat Conduction Dim. Group Biot, Bi Ratio convection at body’s surface/ conduction within the body Equation ℎ𝐿 𝑘 Manipulating into a desired form even further: 𝐿2 𝑇∞ − 𝑇0 𝑥 𝑔0 𝑇 𝑥 − 𝑇0 = − 𝑘 𝐿 2𝑘 1+ ℎ𝐿 Finally: 2𝑘 1+ ℎ𝐿 𝑘 1+ ℎ𝐿 𝑇∞ − 𝑇0 𝑥 𝑔0 𝐿2 𝑇 𝑥 − 𝑇0 = − 1 + 1 𝐵𝑖 𝐿 2𝑘 𝑥 𝑥 − 𝐿 𝐿 1 + 2 𝐵𝑖 1 + 1 𝐵𝑖 2 𝑥 𝑥 − 𝐿 𝐿 2 Differential Equation for Heat Conduction Example! 𝑇∞ − 𝑇0 𝑥 𝑔0 𝐿2 𝑇 𝑥 − 𝑇0 = − 1 + 1 𝐵𝑖 𝐿 2𝑘 1 + 2 𝐵𝑖 1 + 1 𝐵𝑖 Special cases of the problem: I. The Biot Number approaches infinity. In this case, the boundary conditions should have been: 𝑎𝑡 𝑥 = 0, 𝑇(0) = 𝑇0 𝑎𝑡 𝑥 = 𝐿, 𝑇 𝐿 = 𝑇∞ *When Bi approaches infinity, then the heat transfer coefficient, h, approaches infinity also. 𝑥 𝑥 − 𝐿 𝐿 2 Differential Equation for Heat Conduction Example! 𝑇∞ − 𝑇0 𝑥 𝑔0 𝐿2 𝑇 𝑥 − 𝑇0 = − 1 + 1 𝐵𝑖 𝐿 2𝑘 1 + 2 𝐵𝑖 1 + 1 𝐵𝑖 Special cases of the problem: I. The Biot Number approaches infinity. The resulting equation when 𝐵𝑖 → ∞ is: 𝑇∞ − 𝑇0 𝑔0 𝐿2 𝑥 𝑥 𝑇 𝑥 − 𝑇0 = 𝑥+ − 𝐿 2𝑘 𝐿 𝐿 Recall the result when g0 is zero! 2 𝑥 𝑥 − 𝐿 𝐿 2 Differential Equation for Heat Conduction Example! 𝑇∞ − 𝑇0 𝑥 𝑔0 𝐿2 𝑇 𝑥 − 𝑇0 = − 1 + 1 𝐵𝑖 𝐿 2𝑘 1 + 2 𝐵𝑖 1 + 1 𝐵𝑖 Special cases of the problem: II. The Biot Number approaches zero. In this case, the boundary conditions should have been: 𝑎𝑡 𝑥 = 0, 𝑇(0) = 𝑇0 𝑑𝑇 𝑎𝑡 𝑥 = 𝐿, =0 𝑑𝑥 *When Bi approaches zero, then the heat transfer coefficient, h, approaches zero also. 𝑥 𝑥 − 𝐿 𝐿 2 Differential Equation for Heat Conduction Example! 𝑇∞ − 𝑇0 𝑥 𝑔0 𝐿2 𝑇 𝑥 − 𝑇0 = − 1 + 1 𝐵𝑖 𝐿 2𝑘 1 + 2 𝐵𝑖 1 + 1 𝐵𝑖 Special cases of the problem: II. The Biot Number approaches zero. The resulting equation when 𝐵𝑖 → 0 is: 𝑔0 𝐿2 𝑥 𝑥 𝑇 𝑥 − 𝑇0 = 2 − 2𝑘 𝐿 𝐿 Q: What does dT/dx = 0 imply? 2 𝑥 𝑥 − 𝐿 𝐿 2 Differential Equation for Heat Conduction Exercise! A 10-cm diameter nickel-steel sphere has a thermal conductivity, k = 10 W/m-K. Within the sphere, 800 W/m3 of heat is being generated. The surrounding air is at 20°C and the heat transfer coefficient from the surroundings to the surface of the sphere is 10 W/m2-K. What is the temperature at the center of the sphere? Energy Equation Consider a differential volume element: Recall: Combined Energy Flux 1 2 𝒆= 𝜌𝑣 + 𝜌𝑈 𝒗 + 𝝅 ∙ 𝒗 + 𝒒 2 Recall: First Law of Thermodynamics Energy Equation Consider a differential volume element: Rate of Increase in KE and Internal Energy: (Accumulation) Rate of Energy IN – OUT: Rate of Work Done by External Forces, g: Energy Equation Consider a differential volume element: Combining them: Expanding the combined energy flux term… Energy Equation Consider a differential volume element: THE ENERGY EQUATION Energy Equation Consider a differential volume element: The complete form of the Energy Equation Energy Equation Consider a differential volume element: If we subtract the mechanical energy balance from the energy equation: THE EQUATION OF CHANGE FOR INTERNAL ENERGY Energy Equation Consider a differential volume element: If we subtract the mechanical energy balance from the energy equation: THE EQUATION OF CHANGE FOR INTERNAL ENERGY 𝜕 𝐷𝑈 𝜌𝑈 + 𝛻 ∙ 𝜌𝑈𝒗 = 𝜌 𝜕𝑡 𝐷𝑡 Energy Equation Consider a differential volume element: Putting the internal energy in substantial derivative form: By absorbing the pressure force term, U becomes H. Since D𝐻 𝐷𝑡 = 𝐷𝑇 𝜌𝐶𝑝 𝐷𝑡 then at constant pressure: 𝐷𝑇 𝜌𝐶𝑝 = − 𝛻 ∙ 𝑞 − 𝜏 ∙ 𝛻𝒗 𝐷𝑡 Convenient Form! Energy Equation Special Cases of the Energy Equation: 𝐷𝑇 𝜌𝐶𝑝 = − 𝛻 ∙ 𝑞 − 𝜏 ∙ 𝛻𝒗 𝐷𝑡 1. Fluid at constant pressure and small velocity gradients. R: C: S: 𝐷𝑇 𝜌𝑐𝑝 = 𝑘𝛻 2 𝑇 𝐷𝑡 Energy Equation Special Cases of the Energy Equation: 𝐷𝑇 𝜌𝐶𝑝 = − 𝛻 ∙ 𝑞 − 𝜏 ∙ 𝛻𝒗 𝐷𝑡 2. For solids 𝜌𝑐 𝜕𝑇 = 𝑘𝛻 2 𝑇 𝑝 𝜕𝑡 Fourier’s Second Law of Conduction 3. With Heat Generation (simply added) 𝐷𝑇 𝜌𝑐𝑝 = 𝑘𝛻 2 𝑇 + 𝑔 𝐷𝑡 Energy Equation Example! 𝐷𝑇 𝜌𝐶𝑝 = − 𝛻 ∙ 𝑞 − 𝜏 ∙ 𝛻𝒗 𝐷𝑡 A solid cylinder in which heat generation is occurring uniformly as g W/m3 is insulated on the ends. The temperature of the surface of the cylinder is held constant at Tw K. The radius of the cylinder is r = R m. Heat flows only in the radial direction. Using the Energy Equation only, derive the temperature profile at steadystate if the solid has a constant k. Energy Equation Example! 𝐷𝑇 𝜌𝐶𝑝 = − 𝛻 ∙ 𝑞 − 𝜏 ∙ 𝛻𝒗 𝐷𝑡 Using the solids special case with cylindrical coordinates: This can be rewritten as: Energy Equation Example! 𝐷𝑇 𝜌𝐶𝑝 = − 𝛻 ∙ 𝑞 − 𝜏 ∙ 𝛻𝒗 𝐷𝑡 From here on, the solution is just the same as with the electrical wire: