First law of thermodynamics
advertisement

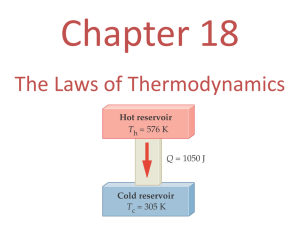
The Laws of Thermodynamics Physics Lecture Notes The Laws of Thermodynamics (01 of 38) Photo © Vol. 05 Photodisk/Getty FOUNDRY: It requires about 289 Joules of heat to melt one gram of steel. In this chapter, we will define the quantity of heat to raise the temperature and to change the phase of a substance. • • • • Sadi Carnot 1796 – 1832 French Engineer Founder of the science of thermodynamics • First to recognize the relationship between work and heat Thermodynamics is the study of processes in which energy is transferred as heat and as work. IThe internal energy is the sum of all the energy of all the molecules in an object: random translational kinetic energy rotational kinetic energy vibrational energy intermolecular energy associated with their bonding. ZEROTH LAW The Zeroth law states that "If bodies A and B are each separately in thermal equilibrium with body C, then A and B are in thermal equilibrium with each other." The common property between A and B is called temperature. Topics 1) The First Law of Thermodynamics 2) Work Done on a Gas 3) Pressure - Volume Graph 4) Thermodynamic Processes 5) The Second Law of Thermodynamics 6) Heat Engines 7) Carnot cycle 8) Entropy The Laws of Thermodynamics (02 of 38) First Law of Thermodynamics System U Q W Environment DU = Q - W The Laws of Thermodynamics (03 of 38) The Ideal Gas Law Volume (m3) Pressure (Pa) PV = NkT Number of Molecules Absolute Temperature (K) Boltzmann’s Constant (1.38 x 10-23 J/K) Temperature and kinetic Theory13 Thermodynamic Processes A. Isobaric Constant Pressure B. Iso-volumetric Constant Volume C. Isothermal Constant Temp. D. Adiabatic No Heat Transfer between systems The Laws of Thermodynamics (06 of 38) Pressure - Volume Graph Isotherms (lines of constant temperature) P Pressure T4 T3 T2 T1 Area under curve represents work Internal energy is proportional to temperature V Volume The Laws of Thermodynamics (05 of 38) HEAT ENGINES Hot Res. TH Qhot Engine Qcold Cold Res. TC Wout A heat engine is any device which through a cyclic process: • Absorbs heat Qhot • Performs work Wout • Rejects heat Qcold THE SECOND LAW OF THERMODYNAMICS Hot Res. TH Qhot Wout Engine Qcold Cold Res. TC It is impossible to construct an engine that, operating in a cycle, produces no effect other than the extraction of heat from a reservoir and the performance of an equivalent amount of work. Not only can you not win (1st law); you can’t even break even (2nd law)! THE SECOND LAW OF THERMODYNAMICS Hot Res. TH Hot Res. TH 400 J 100 J Engine 400 J 400 J Engine 300 J Cold Res. TC • A possible engine. Cold Res. TC • An IMPOSSIBLE engine. EFFICIENCY OF AN ENGINE Hot Res. TH QH W Engine QC The efficiency of a heat engine is the ratio of the net work done W to the heat input QH. e= W = QH Cold Res. TC e=1- Q H - QC QH QC QH EFFICIENCY EXAMPLE Hot Res. TH 800 J W An engine absorbs 800 J and wastes 600 J every cycle. What is the efficiency? QC Engine e=1- 600 J Cold Res. TC 600 J e=1- 800 J ----- QH e = 25% Question: How many joules of work is done? EFFICIENCY OF AN IDEAL ENGINE (Carnot Engine) Hot Res. TH QH Engine QC W maember perfect engine, the quantities Q of heat gained and lost are proportional to TH -temperatures TC the absolute T. e= TH Cold Res. TC e=1- TC TH The Carnot Cycle Th= 550 K For the engine = 890 J Qh Work done by engine each cycle Engine W W Qh - Qc Qc W 890 J - 470 J 420 J = 470 J Tc The efficiency of the engine W 420 J e 0.472 Qh 890 J 47.2 % The Laws of Thermodynamics (26 of 38) The Carnot Cycle Th= 550 K Temperature of the cool reservoir Qc Tc Qh Th Qc Tc Th Qh 470 J Tc 550 K 550 J = 890 J Qh Engine Qc 290 K W = 420 J = 470 J Tc The engine undergoes 22 cycles per second, its mechanical power output J cycles W 22 Wf 420 P cycle s t 9.24 kW The Laws of Thermodynamics (27 of 38) The Carnot Cycle Th A carnot engine absorbs 900 J of heat each cycle and provides 350 J of work Qh The efficiency of the engine Qc W 350 J e 0.389 Qh 900 J = 900 J Engine 38.9 % W = 350 J Tc The heat ejected each cycle W Qh - Qc Qc Qh - W 900 J - 350 J 550 J The Laws of Thermodynamics (28 of 38) The Carnot Cycle Th A carnot engine absorbs 900 J of heat each cycle and provides 350 J of work Qh The engine ejects heat at 10 oC The temperature of the hot reservoir Qc Qc Tc Qh Th = 900 J Engine W = 350 J =550 J Tc = 283 K Qh Th Tc Qc 900 J Tc 283 K 550 J 463 K 190 oC The Laws of Thermodynamics (29 of 38) The Carnot Cycle Th= 650 K A carnot engine operates between a hot reservoir at 650 K and a cold reservoir at 300 K. If it absorbs 400 J of heat at the hot reservoir, how much work does it deliver? Qh = 400 J Engine W=? Qc Th - Tc W e Qh Th Tc= 300 K Th - Tc W Qh Th 650 K - 300 K W 400 J 650 K 215 J The Laws of Thermodynamics (30 of 38) Entropy Entropy is a measure of the disorder of a system. This gives us yet another statement of the second law: Natural processes tend to move toward a state of greater disorder. Example: If you put milk and sugar in your coffee and stir it, you wind up with coffee that is uniformly milky and sweet. No amount of stirring will get the milk and sugar to come back out of solution. The Laws of Thermodynamics (33 of 38) Entropy Another example: when a tornado hits a building, there is major damage. You never see a tornado approach a pile of rubble and leave a building behind when it passes. Thermal equilibrium is a similar process – the uniform final state has more disorder than the separate temperatures in the initial state. The Laws of Thermodynamics (34 of 38) Entropy Another consequence of the second law: In any natural process, some energy becomes unavailable to do useful work. If we look at the universe as a whole, it seems inevitable that, as more and more energy is converted to unavailable forms, the ability to do work anywhere will gradually vanish. This is called the heat death of the universe. The Laws of Thermodynamics (35 of 38) Summary First law of thermodynamics: ΔU Q - W Isothermal process: temperature is constant. Adiabatic process: no heat is exchanged. Work done by gas at constant pressure: W PΔU Heat engine changes heat into useful work (requires temperature difference). Efficiency of a heat engine: Carnot efficiency: QL W e 1QH QH TL ec 1 TH The Laws of Thermodynamics (36 of 38)