powerpoint - University of Illinois at Urbana
advertisement

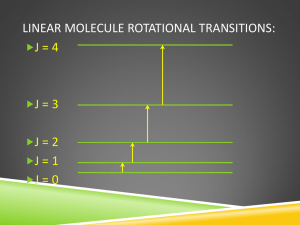
Lecture 33 Rotational spectroscopy: energies (c) So Hirata, Department of Chemistry, University of Illinois at Urbana-Champaign. This material has been developed and made available online by work supported jointly by University of Illinois, the National Science Foundation under Grant CHE-1118616 (CAREER), and the Camille & Henry Dreyfus Foundation, Inc. through the Camille Dreyfus Teacher-Scholar program. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the sponsoring agencies. Rotational spectroscopy In this lecture, we will consider the rotational energy levels. In the next lecture, we will focus more on selection rules and intensities. Diatomic molecules A rigid rotor in 3D = the particle on a sphere l(l +1) El = 2 2mr J (J +1) EJ = 2I 2 2 mA mB 2 r Moment of inertia I = mr = mA + mB 2 Polyatomic molecules Moment of inertia I xx = å m x 2 i i i æ I xx ç I = ç I yx ç çè I zx I xy I yy I zy I xz ö ÷ I yz ÷ ÷ I zz ÷ø COM x åm x = åm i i i i i Moments of inertia æ I xx ç ç I yx ç çè I zx æ I xx ç ç I yx ç çè I zx æ I xx ç ç I yx ç çè I zx I xy I yy I zy I xy I yy I zy I xy I yy I zy I xz ö æ ax ÷ç I yz ÷ ç a y ÷ç I zz ÷ø çè az æ a ö ö x ç ÷ ÷ ÷ = Ia ç ay ÷ ç ÷ ÷ ç ÷ø è az ÷ø Principal axis of rotation Principal moment of inertia æ b ö I xz ö æ bx ö x ÷ç ÷ ç ÷ I yz ÷ ç by ÷ = I b ç by ÷ ÷ç ÷ ç ÷ ç ÷ ç I zz ÷ø è bz ø è bz ÷ø æ c ö I xz ö æ cx ö x ÷ç ÷ ç ÷ I yz ÷ ç c y ÷ = I c ç c y ÷ ÷ç ÷ ç ÷ ç ÷ ç I zz ÷ø è cz ø è cz ÷ø When faced with a symmetric matrix, always look for eigenvalues/eigenvectors. Linear rotors mAmB 2 I a = Ib = R mA + mB One moment of inertia (Ic) equal to zero Spherical rotors 8 I a = I b = I c = mH R2 3 I a = I b = I c = 4mF R Three equal moments of inertia 2 Symmetric rotors I a = 2mH R 2 (1- cosq ) mH mN I b = I c = mH R (1- cosq ) + R 2 (1+ 2cosq ) 3mH + mN 2 Two equal moments of inertia The rotational energy levels n (in cm–1) = v / c = hv / hc = E / hc ( ) 2I = hcBJ ( J +1) EJ = J J +1 B= 2 hc2I = 2 4p cI The rotational constant B is given in cm–1 Quantum in nature Microwave spectroscopy or computational quantum chemistry How could chemists know HF bond length is 0.917Å? Wikipedia mH mF 2 B= ;I= RHF 4p cI mH + mF Spherical & linear rotors In units of wave number (cm–1): ( ) 2I = hcBJ ( J +1) EJ = J J +1 2 F ( J ) BJ J 1 Symmetric rotors Classically, l +l l l -l l l2 æ 1 1 ö 2 E= + = + = +ç la ÷ 2I b 2I a 2I b 2I a 2I b è 2I a 2I b ø Quantum-mechanical rotational terms are 2 b 2 c ( 2 a ) 2 2 a ( 2 a ) ( ) F J , K = BJ J + 1 + A - B K 2 J = 0,1,2,… K = 0,±1,… ,±J 4p cI a 4p cI b Symmetric rotors ( ) ( ) ( ) F J , K = BJ J + 1 + A - B K 2 J = 0,1,2,… K = 0,±1,… ,±J K acts just like MJ. The only distinction is that K refers to rotation around the principal axis, whereas MJ to externally fixed axis Degeneracy F ( J, K ) = BJ ( J +1) + ( A - B) K 2 Linear rotors (2J+1)-fold degeneracy (mJ = 0,±1,…, ±J) Symmetric rotors (2J+1)-fold degeneracy (mJ = 0,±1,…, ±J) another 2-fold degeneracy (K = ±1,…, ±J) [(A – B) ≠ 0 and K and –K give the same energy] Spherical rotors (2J+1)-fold degeneracy (mJ = 0,±1,…, ±J) another (2J+1)-fold degeneracy (K = 0,±1,…, ±J) [(A – B) = 0 and energy does not depend on K] K for shape, mJ for orientation. Zeeman effect mJ-degeneracy can be lifted by applying a static magnetic field along z axis. (0)* ˆ ˆ (0) Y h× l Y dt z ò Zeeman effect First-order perturbation theory (0)* ˆ ˆ (0) Y h× l Y dt z ò E ( J, M J ) = hcBJ ( J +1) - ghM J Stark effect mJ-degeneracy can be lifted by applying a static electric field along z axis. E J , M J hcBJ J 1 a J , M J 2 E 2 aJ,MJ J J 1 3M J2 2hcBJ J 1 2 J 1 2 J 3 Stark effect Second-order perturbation theory E J , M J hcBJ J 1 a J , M J 2 E 2 aJ,MJ J J 1 3M J2 2hcBJ J 1 2 J 1 2 J 3 Linear rotors 2J+1 degenerate No rotation around the axis No degeneracy Zeeman effect 1ST order PT Stark effect 2ND order PT Spherical rotors 2J+1 degenerate 2J+1 degenerate Zeeman effect 1ST order PT Stark effect 2ND order PT Symmetric rotors 2J+1 degenerate Doubly degenerate Zeeman effect 1ST order PT Stark effect 2ND order PT Willis Flygare From UIUC Department of Chemistry website “Willis Flygare earned his bachelor's degree from St. Olaf College in 1958 and his doctorate from the University of California at Berkeley in 1961. He was a professor of chemistry at Illinois from 1961 until his death. During that period he developed a new experimental method involving the molecular Zeeman effect, and with it, he measured most of the known molecular quadrupole moments and magnetic susceptibility anisotropies. He developed a highly sensitive microwave spectrometer by combining molecular beams with Fourier transform techniques.” Summary We have learned the rotational energy levels of molecules in the rigid-rotor approximation. We have classified rigid rotors into linear rotors, spherical rotors, symmetric rotors, and the rest. We have discussed the energy levels and their degeneracy of these rotors. We have learned the Zeeman and Stark effects.