Powerpoint Discussion of Terminology
advertisement

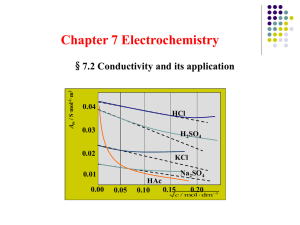
Conductance, resistance, conductivity, and resistivity: a summary for people who weren’t paying attention in PChem or who had PChem long ago by Michael Collins Solution conductivity • Spans a factor of more than 10 million from ultra pure water to concentrated ionic solutions • Remarkably easy to measure with the MicroLab system of FS-522 lab interface, software, and sensor • Determined by the – – – – – Concentration of ions Mobility of ions Temperature Solvent Physical arrangement of electrodes in the conductivity cell Accurate measurements require a bit more than routine measurements • Rugged, reliable conductivity cell needed • Temperature control is essential – Ion mobility can change by up to 10% between 20o and 25oC – Weak electrolytes can change even more depending on the temperature dependence of Keq • High quality distilled or deionized water is a plus – Dissolved CO2 is a weak acid, adds ions! – Dissolved minerals can swamp the conductivity of dilute solutions – In any case, the conductivity of water needs to be measured and can be subtracted to get the net conductivity Terminology and review of concepts • Ohm’s Law: V = IR • V is the applied voltage across the resistor • The current flows in proportion to the voltage for a fixed resistance • Electrical resistance, R, of a conductor: – The measure of the amount of current a circuit can carry for a given voltage – An ideal conductor is a material whose resistance R is a constant over all applied voltages at a given temperature – SI units for electrical circuits: • voltage (aka “potential”) unit is the volt • Current unit is the ampere • resistance unit is the ohm Terminology and review of concepts • Conductance: – The reciprocal of resistance – Symbol is L = 1/R – “Conductance” as a term is not used much in wired circuits but is used extensively in circuits involving solutions – Units are Siemens • Olden days: units were ohm-1 and mho • Present day: unit is the siemen – 1 siemen = 1 ohm-1 = 1 mho – Not to be confused with the Siemens Corp. Terminology and review of concepts • Resistivity (formerly called specific resistance) – A measure of the resistance of a wire of length l (meters) and cross sectional area A (m2) ρ = R*A/ l R = ρ* l/A – ρ is an intensive property of the conductor • Thicker wires have lower resistance • Longer wires have higher resistance • All wires of a given material have the same R*A/ l ! – Units are ohm-meters – In solution measurements • A is the area of the electrodes in the cell • l is the distance separating the electrodes Terminology and review of concepts • Conductivity (formerly called specific conductance) – Defined as the inverse of the resistivity κ = 1/ρ = l/A * 1/R = l/A * L = K * L – Conductivity has the reciprocal SI units of resistivity ohm-1m-1 ≡ siemens.m-1 – K (= l/A) is called the cell constant and is a measure of the area of the electrodes in the measuring cell and the distance between them. • SI unit is m-1 • though it is often given in cm-1 Precise value of K for a given cell must be determined by measuring the conductances of standard solutions of known conductivity Molar conductivity of salt solutions • Λm = κ/c • c is given in SI units (mol/m3) – So units on Λm are – (siemens/m).m3/mol = siemens.m2/mol • Usual units for concentration are mol/L – c (mol/m3) = M (mol/L) x 1L/1dm3 x (10dm/m)3 – c (mol/m3) = 1000M (mol/L) • So Λm = κ/c = κ/(1000M) (siemens.m2/mol) – NOTE: Λm is often given in siemens.cm2/mol – In which case, convert (10 cm)2 = (1m)2 General operational procedure for determining a cell constant • Obtain or prepare aqueous solutions of salt solutions of known conductivity κ using good quality DI or distilled water. – Note the units used in the κ value reported! – Usually SI units of Siemens/m are not used – more typically millisiemens/cm or microsiemens/cm (μS/cm) – Make all measurements with the appropriate units in mind • Fill sample cell/beaker/vial with the same batch of DI or distilled water used to prepare sample and measure the conductance of the water • Rinse and fill cell/beaker/vial with sample of known conductivity and measure its conductance – Subtract conductance of the water from the known conductance to get the net conductance L of the solution – Determine the cell constant K = κ/L • Rinse and fill cell/beaker/vial with unknown sample and measure its conductance – Subtract conductance of the water from the unknown conductance to get the net conductance L of the unknown – Determine the conductivity κ = KL Check out the MicroLab video • Short video showing via screen capture the process of calibrating the conductivity sensor to determine the cell constant and measuring the conductivity of an unknown • INSERT LINK HERE The calibration step relates the solution conductivity to the measured conductance to obtain the cell constant. Conc. NaCl (g/1000.0 mL) Conductivity (µS/cm) 0.000 0.050 0.100 0.150 0.200 0.500 1.000 1.500 2.000 0.05 105 210 315 415 1020 1990 2930 3860 Thus all measurements made with the MicroLab system after calibration are conductivities in µS/cm MicroLab method: • Plug conductivity cell into its jack on the FS-522 lab interface • Make sure the latest version of the MicroLab software is installed on the Windows PC • Connect the FS-522 interface to the PC and turn it on • Open the MicroLab software • Run the default experiment. Then – Add sensor (conductivity) • Choose the range you want to use. 0-20,000 μS/cm for routine use; 0 – 2,000 μS/cm for dilute solutions or weak electrolytes – Choose a new calibration file • This will relate sensor response (L) to known conductivity (κ) – Measure conductance of (each) sample, entering its known conductivity κ (note the units that you use to enter the data – most standards are in μS/cm – Add a regression line and save. • The slope of the line is the the cell constant. Units will be in cm-1. • This will exit you to the main program. – Drag the conductivity sensor to the digital display – Measure the conductivity of your unknown(s) in the same way you did with setting up the calibration file. – Units will be in μS/cm or whatever factor of siemens you used in the calibration. MicroLab method: – Of course you can set up any experiment that you want by adding other sensors. • In a kinetics run, you could add time and measure κ vs. time • In a titration, you could measure κ vs. volume or drops form keyboard or drop counter • In a P Chem experiment you may wish to measure molar conductivity vs. concentration of a weak acid to determine its Ka MicroLab method: – NOTE: conductivity “cell constants” are not actually constant over factors of tens of thousands of μS • Limit is about 2 orders of magnitude – Best technique is to calibrate with standard solutions that span the range of samples you expect – If an exceptionally wide range of conductance is needed, you may wish to use a second order fit to the data for better results, especially in the low ranges Sample calculation 1: determination of a cell constant • You prepare standard NaCl solution to be exactly 2.000 g/L using your local DI water and dried reagent grade NaCl. This solution is reported in the literature to have a known conductivity of 3860 μS/cm at 25oC. • In your conductivity cell at 25oC, DI water has a conductance of 230 μS. • In your conductivity cell 25oC, your solution has a conductance of 4160 μS • What is the cell constant for your cell at 25oC? (see solution on next slide) Sample problem 1: solution • Compute net conductance L by subtracting water’s value from the measured value for the standard: L = (4160 – 230) μS = 3930 μS • Calculate the cell constant K K = κ/L = (3860μS/cm)/(3930μS) K = 0.982 /cm Sample problem 2: computing conductivity from a conductance measurement • A solution has a conductance of 3620 μS at 25oC in your conductivity cell. What is its conductivity? Sample problem 2: solution The cell constant K = 0.982/cm (from problem 1) The conductance L = 3620 μS The conductivity is κ=KxL= κ = 0.982 /cm x 3620 μS κ = 3560 μS/cm This can be converted to SI units κ = 3560 μS/cm x 100 cm/m x 10-6 siemens/μS κ = 0.356 siemens/m (3 sig fig) Sample problem 3: A 0.0100M KCl solution is found to have a conductivity of 1.410 mS/cm at 25oC. What is the molar conductivity of the KCl in the solution in the usual units of siemens.cm2/mol? Sample problem 3 solution: Λm = κ/c = κ/(1000M) (siemens.m2/mol) κ = [(1.410 mS/cm) x (10-3siemen/mS) x 102cm/m κ = 0.141 siemens/m Λm = κ/(1000M) = 0.141/(1000 x 0.01) = 0.0141 siemens.m2/mol Now convert Λm from SI into siemens.cm2/mol: Λm = 0.0141 siemens.m2/mol x (100 cm/m)2 = Λm = 141 siemens.cm2/mol Good luck! • And have fun!