Energy, Work and Heat
advertisement

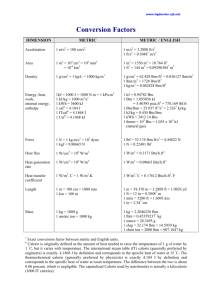
Lesson 3 ENERGY, WORK, AND HEAT • DEFINE the following: – – – – Heat Latent heat Sensible heat Units used to measure heat • DEFINE the following thermodynamic properties: – Specific enthalpy – Entropy Energy • The capacity of a system to perform work or produce heat • Categories – – – – – – – – Potential Energy Kinetic Energy Specific Internal Energy Specific P-V Energy Enthalpy Work Heat Entropy Potential Energy • Energy of position • PE = mgz/gc where PE = potential energy (ft-lbf) m = mass (lbm) z = height above some reference level (ft) g = acceleration due to gravity (32.17 ft/sec2) gc = gravitational constant = 32.17 ft-lbm/lbf-sec2 Kinetic Energy • Energy of motion • KE = mv2/2gc where: KE = kinetic energy (ft-lbf) m = mass (lbm) v = velocity (ft/sec) gc = gravitational constant = 32.17 ft-lbm/lbf-sec2 Specific Internal Energy • Internal energy per unit mass • Equal to total internal energy (U) divided by the total mass (m). u = U/m where: u = specific internal energy (Btu/lbm) U = internal energy (Btu) m = mass (lbm) • Internal energy includes – Energy due to rotation, vibration, translation, and interactions among molecules – Can not be measured or evaluated directly Specific P-V Energy • Energy per unit mass. • Equals the total Pressure times the Volume of a liquid divided by the total mass m • Also equals the product of the pressure P and the specific volume v, and is written as Pv Pv = PV/m where: P = pressure (lbf/ft2) V = volume (ft3) n = specific volume (ft3/lbm) V m m = mass (lbm) Enthalpy • A property of a substance, like pressure, temperature, and volume, • Cannot be measured directly • Normally given with respect to some reference value. • Usually used in connection with an "open" system problem in thermodynamics • Specific enthalpy (h) h = u + Pv where u is the specific internal energy (Btu/lbm) P is the pressure of the system (lbf/ft2) v is the specific volume (ft3/lbm) of the system. Work • Energy in transit • Not a property of a system – it is a process done by or on a system • Defined as the action of a force on an object through a distance W = Fd where: W = work (ft-lbf) F = force (lbf) d = displacement (ft) Heat • Like work, is energy in transit • Occurs at the molecular level as a result of a temperature difference • Denoted by the letter Q • Heat transferred per unit mass denoted by q q = Q/m where: q = heat transferred per unit mass (Btu/lbm) Q = heat transferred (Btu) m = mass (lbm) Heat (continued) • Sensible Heat - The heat added to or removed from a substance to produce a change in its temperature • Latent Heat - The amount of heat added to or removed from a substance to produce a change in phase. – Latent Heat of Fusion - the amount of heat added or removed to change phase between solid and liquid. – Latent Heat of Vaporization - the amount of heat added or removed to change phase between liquid and vapor Heat Capacity • The ratio of the heat (Q) added to or removed from a substance to the change in temperature (ΔT) produced • Denoted by Cp • Specific heat (cp) is heat capacity of a substance per unit mass • Applies when the heat is added or removed at constant pressure Heat Capacity (continued) Cp = Q/ΔT cp = Q/mΔT cp = q/ΔT where: Cp = heat capacity at constant pressure (Btu/°F) cp = specific heat at constant pressure (Btu/lbm-°F) Q = heat transferred (Btu) q = heat transferred per unit mass (Btu/lbm) m = mass (lbm) ΔT = temperature change (°F) Entropy (S) • A property of a substance • Quantifies the energy of a substance that is no longer available to perform useful work ΔS = ΔQ/Tabs Δ s = Δq/Tabs where: Δ S = the change in entropy of a system during some process (Btu/°R) ΔQ = the amount of heat transferred to or from the system during the process (Btu) Tabs = the absolute temperature at which the heat was transferred (°R) Δs = the change in specific entropy of a system during some process (Btu/lbm -oR) Δq = the amount of heat transferred to or from the system during the process (Btu/lbm) Power • Power - The time rate of doing work. • Units are energy per unit time – English system - ft-lbf/sec or ft-lbf/hr – Horse Power - hp – British thermal units per hour - Btu/hr – Electrical units - watts (W) or kilowatts (kW) Energy and Power Equivalencies • • • • • • • • • 1 ft-lbf = 1.286 x 10-3 Btu = 3.766 x 10-7 kW-hr 1 Btu = 778.3 ft-lbf = 2.928 x 10-4 kW-hr 1 kW-hr = 3.413 x 103 Btu = 2.655 x 106 ft-lbf 1 hp-hr = 1.980 x 106 ft-lbf 1 Joule = 778 ft lbf/Btu 1 ft-lbf/sec = 4.6263 Btu/hr = 1.356 x 10-3 kW 1 Btu/hr = 0.2162 ft-lbf/sec = 2.931 x 10-4 kW 1 kW = 3.413 x 103 Btu/hr = 737.6 ft-lbf/sec 1 hp = 550.0 ft-lbf/sec