Lecture 32
advertisement

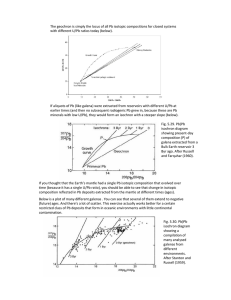
Re-Os & U-Th-Pb Isotope Geochemistry Lecture 32 The Re-Os System • • • • • • 187Re decays to 187Os by β– decay with a half-life of 42 billion years. Unlike the other decay systems of geological interest, Re and Os are both siderophile elements: they are depleted in the silicate Earth and presumably concentrated in the core. The resulting very low concentration levels (sub-ppb) make analysis extremely difficult. Interest blossomed when a technique was developed to analyze OsO4– with great sensitivity. It remains very difficult to measure in many rocks, however. Peridotites have higher concentrations. The siderophile/chalcophile nature of these elements, making this a useful system to address questions of core formation and ore genesis. Os is a highly compatible element (bulk D ~ 10) while Re is moderately incompatible and is enriched in melts. For example, mantle peridotites have average Re/Os close to the chondritic value of 0.08 whereas the average Re/Os in basalts is ~10. Thus partial melting appears to produce an increase in the Re/Os ratio by a factor of >102. As a consequence, the range of Os isotope ratios in the Earth is very large. The mantle has a 187Os/188Os ratio close to the chondritic value of, whereas the crust appears to have a a 187Os/188Os > 1. By contrast, the difference in 143Nd/144Nd ratios between crust and mantle is only about 0.5%. The near chondritic a 187Os/188Os of the mantle is surprising, given that Os and Re should have partitioned into the core very differently. This suggests most of the noble metals in the silicate Earth are derived from a late accretionary veneer added after the core formed. In addition, 190Pt decays to 186Os with a half-life of 650 billion years. The resulting variations in 186Os/188Os are small. Os Isotopes in the SCLM • Since the silicate Earth appears to have a nearchondritic 187Os/188Os ratio, it is useful to define a parameter analogous to εNd and εHf that measures the deviation from chondritic. γOs is defined as: - ( Os Os ) Os ) g ´100 Os ( Os) Studies of pieces of subcontinental lithospheric Os • • • ( = 187 Os 188 187 sample 187 188 188 Chond Chond mantle xenoliths show that much of this mantle is poor in clinopyroxene and garnet and hence depleted in its basaltic component. Surprisingly, these xenoliths often show evidence of incompatible element enrichment, including high 87Sr/86Sr and low ε . This latter feature is often Nd attributed to reaction of the mantle lithosphere with very small degree melts percolating upward through it (a process termed “mantle metasomatism”). This process, however, apparently leaves the Re-Os system unaffected, so that 187Re/188Os and 187Os/188Os remain low. Low γOs is a signature of lithospheric mantle. Os Isotopes in Seawater • Os isotopes in seawater (tracked by measuring Os in Mn nodules and black shales) reveals a variation much like that of 87Sr/86Sr. • The reflects a balance of mantle and crustal inputs. • And, perhaps, meteoritic ones. Very low ratios occur at the K-T boundary. Ratio was already decreasing before then: Deccan traps volcanism? (supports the hit ‘em while their down theory of the K-T extinction). U-Th-Pb • In the U-Th-Pb system there are three decay schemes producing 3 isotopes of Pb. Two U isotopes decay to 2 Pb isotopes, and since the parent and daughter isotopes are chemically identical, we get a particularly powerful tool. • Following convention, we will designate the 238U/204Pb ratio as μ, and the 232Th/238U ratio as κ. We can write two versions of our isochron equation: Pb æ = 204 Pb çè 206 206 204 Pb ö + µ(el238t -1) ÷ Pb ø 0 235 Pb æ 207 Pb ö U = ç 204 ÷ + µ 238 (el238t -1) 204 Pb è Pb ø 0 U 207 o Conventionally, the 235U/238U was assumed to have a constant, uniform value of 1/137.88. Recent studies, however, have demonstrated that this ratio varies slightly due to kinetic chemical fractionation. Consequently, for highest precision, it should be measured. In most cases, however, we can use the revised apparent average value of 1/137.82. Pb-Pb isochrons • These equations can be rearranged by subtracting the initial ratio from both sides. For example: Pb = µ(el238t -1) 204 Pb 206 ∆ • Dividing the two: ∆ 207 Pb / 204 Pb = ∆ 206 Pb / 204 Pb o • • • U (el238t -1) 238 U (el235t -1) 235 the 235U/238U is the present day ratio and assumed constant. The left is a slope on a plot of 207Pb/204Pb vs 206Pb/204Pb. Slope is proportional to time, and so is an isochron. The value is that we need not know or measure the U/Pb ratio (which is subject to change during weathering). In essence, we have 3 dating methods: 235U-207Pb, 238U-206Pb and PbPb. An age is said to be concordant when all 3 agree. Pb Isotopic Evolution • • • Because the half-life of 235U is much shorter than that of 238U, 235U decays more rapidly and Pb isotopic evolution follows curved paths on this plot. o The exact path depends upon µ. o True only for the planet as a whole, not individual rock formations. Parts of the planet can lie to the right if U/Pb increased or the the left if U-Pb decreased. All systems that begin with a common initial isotopic composition at time t0 lie along a straight line today. This line is the Pb-Pb isochron. When the solar system formed 4.57 billion years ago, it had a single, uniform Pb isotope composition. All closed systems, such as planets, that started with this value at that time must lie on a 4.57 Ga isochron, called the Geochron. o • The Earth as a whole must fall on this line if it formed at the same time as the solar system with the solar system initial Pb isotopic composition. o o The problem is that Earth may be 100 Ma younger than the ‘solar system’ - because it took a long time to form large terrestrial planets. There is some flexibility in the exact position of the Geochron because the age is not exactly known. 232Th-208Pb • We can combine the growth equations for 208Pb/204Pb and 206Pb/204Pb in a way similar to our 207Pb-206Pb isochron equation We end up with: ∆ 208 Pb / 204 Pb (el238t -1) = k l235t ∆ 206 Pb / 204 Pb (e -1) o where κ is the 232Th/238U ratio. • The left is a slope on a plot of 208Pb/204Pb vs 206Pb/204Pb and is proportional to t and κ. o assuming κ has been constant (except for radioactive decay). Pb Isotope Ratios in the Earth Pb Isotope Ratios in the Earth • • • • • • • Major terrestrial reservoirs, such as the upper mantle (represented by MORB), upper and lower continental crust, plot near the Geochron between growth curves for µ = 8 and µ = 8.8, suggesting µ of the Earth ≈ 8.5. If a system has experienced a decrease in U/Pb at some point in the past, its Pb isotopic composition will lie to the left of the Geochron; if its U/Pb ratio increased, its present Pb isotopic composition will lie to the right of the Geochron. U is more incompatible than Pb, so incompatible element depleted reservoirs should plot to the left of the Geochron, enriched ones to the right. From the other isotopic ratios, we would have predicted that continental crust should lie to the right of the Geochron and the mantle to the left. Surprisingly, Pb isotope ratios of mantle-derived rocks also plot mostly to the right of the Geochron. This indicates the U/Pb ratio in the mantle has increased, not decreased as expected. This phenomenon is known as the Pb paradox and it implies that a simple model of crust–mantle evolution that involves only transfer of incompatible elements from crust to mantle through magmatism is inadequate. There is also perhaps something of a mass balance problem - since everything should average out to plot on the Geochron.