Chapter 17: Molecular Interactions
advertisement

Atkins & de Paula:
Atkins’ Physical Chemistry 9e
Chapter 17: Molecular Interactions
Chapter 17: Molecular Interactions
ELECTRIC PROPERTIES OF MOLECULES
17.1 Electric dipole moments
electric dipole, two electric charges +q and –q separated by a distance R.
electric dipole moment, μ, the vector that points from –q to + q with magnitude μ = qR.
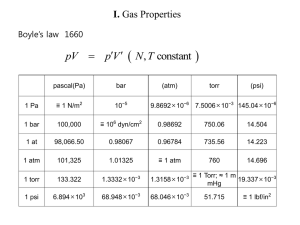
1 D = 3.33564 × 10-30 Cm
Chapter 17: Molecular Interactions
polar molecule, a molecule with a permanent electric dipole moment.
nonpolar molecule, a molecule without a permanent electric dipole moment.
Chapter 17: Molecular Interactions
Resultant electric dipole moments, μres2 = μ12 + μ22 + 2μ1μ2 cos θ.
Chapter 17: Molecular Interactions
Calculation of electric dipole moments, μ2 = μx2 + μy2 + μz2 (μi =Σqjij)
μ = 2.7 D
Chapter 17: Molecular Interactions
Self-test; calculation of the μ of formaldehyde
Chapter 17: Molecular Interactions
17.2 Polarizabilities
induced dipole moment, μ*, the dipole moment induced by an applied electric field.
polarizability, the constant of proportionality α in μ* = αE. (unit = C2 m2 J-1)
polarizability volume, α = α/4πε0. (unit of ε0: C2 m-1 J-1)
polarizability, perturbation expression.
2
z ,0 n
2
n 0 En E0
, where z ,0 n : transition in thez - direction
En E0 mean valueE (HOMO- LUMOgap)
μz,0 n eR
α increases as the molecular size increases
α increases as the HOMO-LUMO gap decreases
e2 R 2
2
E
E
α ≈ R3
e2
4 0 R
Chapter 17: Molecular Interactions
17.3 Polarization
polarization, P, the electric dipole moment density, P = μN.
dielectric, a polarizable, nonconducting medium.
μ = 0, where no electric field is applied
μz = μ, where a strong electric field is applied
μz = μ2E/3kT, where a weak electric field is applied
dp
e E ( ) / kT sin d
e
E ( ) / kT
x E / kT , E ( ) E cos ; energyof dipole
; probability of dipole orientat ion
sin d
0
z cosdp cosdp
e
0
x cos
e
cos sin d
y cos , dy sin d
z
x cos
sin d
x
e
1
xy
dy
e e
x
x
z L( x)
x
1
ye
1
xy
dy
e e
x
e x ex 1
L( x) x x
e e
x
e x 1 x 1 x 2 1 x 3
x
e e
x2
ye xy dy
1
1
e
1
0
1
1
x
x
; Langevinfunct ion
1
2
6
x
L( x) 13 x z
2E
3kT
xy
dy
Chapter 17: Molecular Interactions
orientation polarization, the polarization arising from the permanent dipole moments; is
lost at microwave frequency
distortion polarization, the polarization arising from the distortion of the positions of
the nuclei by the applied field; is lost at IR frequency
electronic polarizability, the polarizability due to the distortion of the electron
2
distribution; is still alive at Vis frequency
z ,0 n
2
frequency dependence of polarizabilities
n 0 En E0
2
ħωn0 = En – E0
2 n 0 z , 0 n
( ) 2
n n 0 2
n0
( )
( )
2
2
z ,0 n
2 n0
2
n
z ,0 n 0 as
2 n0
2
n
Chapter 17: Molecular Interactions
17.4 Relative permittivities
permittivity, the quantity ε in the Coulomb potential energy, V = q1q2/4πεr.
relative permittivity (dielectric constant), εr = ε/ε0. (ε0 = vacuum permittivity)
Debye equation, (εr – 1)/(εr + 2) = ρPm/M.
molar polarization, Pm = (NA/3ε0)(α + μ2/3kT).
Clausius–Mossotti equation, (εr – 1)/(εr + 2) = ρNAα/3Mε0.; no contribution from permanent dipole, μ
Nonpolar molecules or high frequency of applied field
N A 2
slope
9 0 k
Example 17.2
εr = C/C0
Pm
Debye eqn.
refractive index and relative permittivity, nr = εr1/2.
refractive index, nr = c/c. c: speed of light in vacuum, c: speed of light in medium
intercept
μ
N A
3 0
α
Chapter 17: Molecular Interactions
INTERACTIONS BETWEEN MOLECULES
van der Waals interaction, an interaction between closed-shell molecules that varies
with separation as 1/r6.
17.5 Interactions between dipoles
multipole, an array of point charges.
n-pole, an array of point charges with an n-pole moment
but no lower moment.
monopole, a point charge.
quadrupole, an array of point charges that has neither
net charge nor dipole moment.
octupole, an array of point charges that sum to zero and
which has neither a dipole moment nor a quadrupole
moment.
Chapter 17: Molecular Interactions
multipole–multipole potential energy, V 1/rn+m-1.
Chapter 17: Molecular Interactions
,
17.5 (a) The potential energy of interaction
point dipole, a dipole in which the separation between the charges is much smaller
than the distance at which the dipole is being observed; l << r
point dipole-point charge interaction
V
1q2
4 0 r 2
1 q1q2
q1q2 x l / 2 r
qq
1
1
1 2
4 0 r 12 l r 12 l
4 0 r 1 x 1 x
1
1
l r x 1
1 x x2
1 x x2
1 x
1 x
qq
2 xq1q2
qq l
q
1 q1l
V 1 2 (1 x ) (1 x )
1 2 2
1 2 2
4 0 r
4 0 r
4 0 r
4 0 r
V
Chapter 17: Molecular Interactions
,
Calculating the interaction energy of two dipoles
1 2
V
2 0 r 3
1 q1q2 q1q2 q1q2 q1q2 x l / r
q1q2 1
1
2
4 0 r l
r
r
r l
4 0 r 1 x
1 x
1
1
l r x 1
1 x x2
1 x x2
1 x
1 x
2 x 2 q1q2
V
1 23
4 0 r
2 0 r
V
Self-test 17.4
Chapter 17: Molecular Interactions
,
17.5 (b) Dipole-dipole interactions
electric field of point charge, E = q/4πε0r2.
electric field of point dipole, E = μ/2πε0r3.
potential energy of two parallel point dipoles
1 2 f ( )
V
3
4 0 r
[ f(θ) = 1 – 3 cos2 θ ]
See Further information 17.1
Chapter 17: Molecular Interactions
,
Keesom interaction, the interaction of two freely rotating point dipoles:
first contribution to the vdW interaction
C
212 22
V 6 C
3
r
3(4 0 ) 2 kT
<V> = μ1μ2<f>/ 4πε0r
<f>; weighting factor in the averaging;
probability that a particular orientation
will be adopted by a dipole
p e-V/kT, V=μ1μ2 f/4πε0r3
p 1- V/kT + ∙∙∙, when V ‹‹ kT
f
fpd
0
d
1
fe
0
V / kT
d
1
f (1 V / kT )d
0
0
f
1
0
1
fd
0
1
f (V / kT )d
0
1
fd
0
1 2 1 2
fd
f d f
4 0 kTr 3 0
0
1 2
f 2 d
3
0 4 0 kTr
1
1 2
f2
3
4 0 kTr
0
, where 0 denotesan unweightedsphericalaverage
f
0
12 22 f 2
212 22
(1 3 cos ) sin d 0 V
V
0
(4 0 ) 2 kTr
3(4 0 ) 2 kTr 6
1
2
0
6
f2
0
23
Chapter 17: Molecular Interactions
,
C
212 22
Keesom interaction, the interaction of two rotating point dipoles: V 6 C
2
r
3
(
4
)
kT
0
first contribution to the vdW interaction
Negative sign: the average interaction is attractive.
V 1/r6 : a van der Waals interaction.
V 1/T : the greater thermal motion overcomes the
dipole interactions at higher temperatures.
V 1/r6 : arises from V 1/r3 weighted by the energy
in the Boltzmann term ( 1/r3)
Chapter 17: Molecular Interactions
,
17.5 (c) Dipole-induced-dipole interactions
C
V 6
r
12 2
C
4 0
Independent on the temperature;
thermal motion has no effect on the
averaging process
Chapter 17: Molecular Interactions
,
17.5 (d) Induced-dipole-induced-dipole interactions
dispersion interaction (London interaction)
London formula
C
V 6
r
I1 I 2
C 1 2
I1 I 2
3
2
Chapter 17: Molecular Interactions
,
17.5 (e) Hydrogen bonding
hydrogen bond, an attractive interaction between two species that arises from a link
of the form A–H∙∙∙B, where A and B are highly electronegative elements (N, O, or F)
and B possesses a lone pair of electrons.
= c 1 A + c2 H + c3 B
anti-bonding
Net effect: lowering of energy
nonbonding
bonding
Chapter 17: Molecular Interactions
,
17.5 (f) The hydrophobic interaction
hydrophobic, water-repelling; possessing a positive Gibbs energy of transfer from a
nonpolar to a polar solvent. ΔtransferG > 0, ΔtransferH < 0, ΔtransferS < 0
hydrophobicity constant, π = log(S/S0)
S: ratio of the molar solubility of R-A in octanol to that in water
S0: ratio of the molar solubility of H-A in octanol to that in water
hydrophobic interaction, an effective interaction that is due to the increase in entropy of
the surrounding solvent.
A hydrocarbon molecule in a water cage
Chapter 17: Molecular Interactions
,
17.5 (g) The total attractive interaction
total attractive interaction between rotating molecules; dipole-dipole, dipole-induceddipole, and dispersion interactions. V = –C6/r6
limitation of V = –C6/r6; consider only dipolar interactions, assume freely rotating
molecules, and consider only the interactions of pairs of molecules
Axilrod–Teller formula, total dispersion energy of three closed-shell molecules
C6 C6 C6
C
V 6 6 6
rAB rBC rCA rAB rBC rCA 3
C = a(3 cos θA cos θB cos θC + 1), where a ≈ 3/4αC6
Chapter 17: Molecular Interactions
Interactions between dipoles; impact on medicine (molecular
recognition & drug design). See I17.1
Chapter 17: Molecular Interactions
,
17.6 Repulsive and total interactions
hard-sphere potential, V = for r d; V = 0 for r > d.
Mie potential, V = Cn/rn – Cm/rm.
Lennard-Jones potential, V = 4ε{(r0/r)12 – (r0/r)6}. ε:depth of the well, r0:seperation
exp–6 potential, V = 4ε{e–r/r0 – (r0/r)6}.; better than L-J(12,6) potential
where V=0
Chapter 17: Molecular Interactions
GASES AND LIQUIDS
17.7 Molecular interactions in gases
molecular beam, a collimated, narrow stream of molecules travelling though an
evacuated vessel.
hydrodynamic flow, net flow arising from intermolecular collisions.
molecular flow, collision-free flow.
Chapter 17: Molecular Interactions
Chapter 17: Molecular Interactions
supersonic, a stream of molecules in which the average speed of the molecules is much
greater than the speed of sound for the molecules that are not part of the stream.
supersonic beam, a beam obtained when the region of hydrodynamic flow is skimmed
from a supersonic jet and the excess gas pumped away.
crossed beam technique, a technique in which two molecular beams are incident at right
angles.
Low translational T
Chapter 17: Molecular Interactions
differential scattering cross-section, σ, the constant of proportionality between the
change in intensity (dI) and the intensity of the incident beam (I), the number density of
target molecules (N), and the infinitesimal path length dx through the sample: dI = σIN dx.
impact parameter, b, the initial perpendicular separation of the paths of the colliding
molecules.
Chapter 17: Molecular Interactions
Scattering patterns depend on the impact parameter (b) for the impact of two hard
spheres
Chapter 17: Molecular Interactions
for real molecules; scattering patterns depend on the intermolecular potential, molecular
shape, and relative speed of approach as well as the impact parameter.
repulsive core
long range attractive
potential
Chapter 17: Molecular Interactions
quantum oscillation, the modification of the scattering in the forward direction by
interference between the wavefunctions of a particle along two different paths.
rainbow scattering, strongly enhanced scattering in a nonforward direction.
rainbow angle, θr, the angle for which dθ/db = 0 and the scattering is strong.
van der Waals molecules, complexes of the form AB in which A and B are held
together by van der Waals forces or hydrogen bonds.
Chapter 17: Molecular Interactions
17.8 The liquid–vapour interface
17.8 (a) surface tension
surface tension, γ, the constant of proportionality between the increase in surface area of
a liquid and the work needed to create the increase: dw = γdσ (=dA, where constant T).
dA < 0 (dσ < 0); spontaneous process: surfaces have a natural tendency to contract.
Example17.4
dw =2γlh
Chapter 17: Molecular Interactions
17.8 (b) curved surfaces
bubble, a region in which a vapour is trapped by a thin film.
cavity, a vapour-filled hole in a liquid.
droplet, a small volume of liquid
Laplace equation, pin = pout + 2γ/r.
outward force; pressure × area = 4πr2pin
inward force; force from pout & surface tension
force from pout ; pressure × area = 4πr2pout
force from surface tension; 8πγr
dσ = 4π(r+dr)2 - 4πr2 = 8πrdr
dw = 8πγrdr
4πr2pin = 4πr2pout+ 8πγr
pin = pout + 2γ/r
Chapter 17: Molecular Interactions
17.8 (c) capillary action
capillary action, the tendency of liquids to rise up capillary tubes.
capillary rise and surface tension, γ = (ρβ –ρα)ghr/2
Same pressure at same height in a same phase
P1=P6, P2=P5, P2=P3 P5=P3
Curved surface: P4 < P5=P3 P4 < P3
;capillary rise
At equilibrium
P1=P6, P2=P3
P8=P5, P3=P4 P8-P3= P5-P4 P8-P2= P5-P4
P8-P2= (P5-P7)+(P7-P4)
P8-P2=-ραgh, P7-P4 =-ρβgh, P5-P7=2γ/r (θc=0)
-ραgh = 2γ/r - ρβgh
γ = (ρβ –ρα)ghr/2
Chapter 17: Molecular Interactions
nonzero angle between the edge of meniscus and the wall; γsg = γsl+ γlg cos θc
contact angle and interfacial tension, cos θc = (γsg – γsl)/γlg.
superficial work of adhesion, wad = γsg + γlg – γsl (work of adhesion/area of contact)
cos θc = wad/γlg – 1
criterion for surface wetting, 1< wad /γlg < 2.
criterion for non-surface wetting, 0< wad /γlg < 1.
Chapter 17: Molecular Interactions
17.9 Surface films; will be covered in Chap. 18
17.10 Condensation
Kelvin equation for the vapour pressure of droplets, p = p* e2γVm/rRT
supersaturated phase, a phase that is thermodynamically unstable with respect to the
liquid.
spontaneous nucleation centre, a location at which a sufficiently large number of
molecules congregate into a droplet.
nucleate, provide surfaces to which molecules can attach and thereby induce
condensation.
superheated, a liquid that has not boiled but is above its boiling temperature.
supercooled, a liquid that has not frozen but is below its freezing temperature.
Chapter 17: Molecular Interactions
Impact on nanotechnology
Spontaneous Assembly of a Monolayer of Charged Gold Nanocrystals at the Water/Oil Interface
Angew. Chem. Int. Ed. 2004, 43, 458.
Directing Self-Assembly of Nanoparticles at Water/Oil Interfaces
Angew. Chem. Int. Ed. 2004, 43, 5639.