Clinical Pharmacology of Boceprevir (BOC)
advertisement

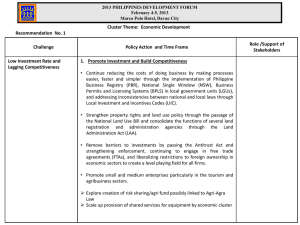
Clinical Pharmacology of Boceprevir: Metabolism, Excretion, and Drug-Drug Interactions C Kasserra, E Hughes, M Treitel, S Gupta, and E O'Mara International Conference on Viral Hepatitis April 11, 2011 Baltimore, MA Disclosure Statement • Full time employee of Merck and Co. o own stock options Boceprevir: Background • Boceprevir (SCH 503034, BOC) Protease inhibitor of the ketoamide class Binds reversibly to active site of HCV NS3 protease to inhibit HCV replication (IC90 400 nM) o Phase 3 trials in treatment-naive and treatmentexperienced genotype 1 HCV patients complete o o highly favourable, statistically significant increases in SVR o Dose: 800 mg TID with food • currently in regulatory review HCV, hepatitis C virus; TID, three times a day. Boceprevir Absorption, Metabolism, Excretion (1) • Absorption o Rapidly absorbed: median Tmax ~2 hrs o Less than dose proportional increases in steady state exposure o No accumulation o Food increases exposure ~40% - 60% o P-gp substrate • Metabolism & Excretion o Extensively metabolized by two distinct pathways Aldo keto reductase (AKR) CYP3A4/5 o single dose of 14C-radiolabeled BOC metabolized to one primary ketone-reduced metabolite radioactivity in urine & feces accounted for ~ 9% and 79% of dose only 3% and 8% as parent in urine and feces respectively. Boceprevir Absorption, Metabolism, Excretion (2) • Metabolism & Excretion (cont’d) Mean plasma t½ of ~3.4 hours Primary route of excretion is hepatic/fecal • Selectivity o Strong reversible CYP3A4 inhibitor o Not a CYP450 isoenzyme inducer o Not a P-gp inhibitor o o Drug-Drug Interaction Studies Boceprevir As Victim Co-administered Drug AKR inhibitors CYP3A4/P-gp inhib CYP3A4 inducer Other Ibuprofen Diflunisal Ketoconazole Ritonavir Clarithromycin* Efavirenz Tenofovir Peginterferon -2b Ribavirin Boceprevir As Perpetrator Co-administered Drug CYP3A4 Midazolam substrate Drospirenone/ Ethinyl estradiol Other Efavirenz Tenofovir Peginterferon -2b Ribavirin‡ Diflunisal DIF 250 mg BID BOC 800 mg TID N = 5 healthy subjects Days: 0 2 4 Treatment 8 6 LS Meana 10 12 Ratio Estimate, % (90% CI) Effect of DIF (250 mg BID) on BOC (800 mg TID) Cmax (ng/mL) BOC BOC + DIF 2259 1936 86 (56–132) AUC(T) (ng·h/mL) BOC BOC + DIF 6868 6597 96 (79–117) Cmin (ng/mL) BOC BOC + DIF 83 108 131 (104–165) aModel-based (least squares) geometric mean; ANOVA extracting the effects due to treatment and subject. AUC(T), area under the plasma concentration versus time curve from time 0 dosing interval; BID, two times a day; BOC, boceprevir; CI, confidence interval; Cmax, maximum observed plasma concentration; Cmin, minimum observed plasma concentration; DIF, diflunisal; LS, least squares; TID, three times a day. Ketoconazole BOC 400-mg single dose on day 1 Day: 1 KET 400 mg BID days 1–6 + BOC 400-mg single dose on day 4 4 1 4 6 N = 12 healthy subjects Boceprevir + Ketoconazole Boceprevir 800 600 BOC + KET vs BOC ratio estimates (90% CI) Mean Concentration 400 of Boceprevir AUC(tf) 231% (200–267) Cmax 141% (100–199) Cmin N/A 200 0 0 12 24 36 48 60 72 Time (h) AUC(tf), area under the plasma concentration versus time curve to the final measurable sampling time; BID, two times a day; BOC, boceprevir; CI, confidence interval; KET, ketoconazole; TID, three times a day. Ritonavir BOC 400 mg TID Day 1 BOC 400 mg TID* + RTV 100 mg QD *BOC stopped at day 15 Day 6 Day 17 N = 16 healthy subjects 1200 BOC (400 mg TID) BOC (400 mg TID) + RTV (100 mg QD) 1000 BOC + RTV (100 mg QD) vs BOC ratio estimates (90% CI) AUC(T) 81% (73–91) Cmax 73% (57–93) Cmin 104% (62–175) 800 Boceprevir (ng/mL) 600 400 200 0 0 2 4 6 8 10 12 Time (h) AUC(T), area under the plasma concentration versus time curve from time 0 dosing interval; BID, two time a day; BOC, boceprevir; CI, confidence interval; RTV, ritonavir; TID, three times a day. Efavirenz Days 1–5: BOC 800 mg TID Day 6: BOC 800 mg single dose Washout ≥7 days N = 12 healthy volunteers Days 1–10: • EFV 600 mg QD Treatment LS Days 11–15: BOC 800 mg TID Day 16: BOC 800 mg single dose Days 11–16: EFV 600 mg QD Meana Ratio Estimate, % (90% CI) Effect of EFV (600 mg QD) on BOC (800 mg TID) Cmax (ng/mL) BOC BOC + EFV 2038 1871 92 (78–108) AUC(0-8h) (ng·h/mL) BOC BOC + EFV 6913 5630 81 (75–89) Cmin (ng/mL) BOC BOC + EFV 94.4 52.5 56 (42–74) Effect of BOC (800 mg TID) on EFV (600 mg QD) Cmax (ng/mL) EFV EFV + BOC 4573 5077 111 (102–120) AUC(0-24h) (ng·h/mL) EFV EFV + BOC 78667 94655 120 (115–126) aModel-based (least squares) geometric mean; ANOVA extracting the effects due to treatment and subject. AUC, area under the plasma concentration-time curve; BOC, boceprevir; CI, confidence interval; Cmax, maximum observed plasma concentration; Cmin, minimum observed plasma concentration; EFV, efavirenz; LS, least squares; QD, once daily; TID, three times a day. Midazolam MDZ 4 mg MDZ 4 mg MDZ 4 mg MDZ 4 mg BOC 800 mg TID –1 1 Days 6 13 8 N = 12 healthy volunteers Treatment LS Mean Ratio Estimate, % (90% CI) Effect of BOC (800 mg TID) on MDZ (4-mg single doses) Cmax ng/mL AUC(0-12hr) (ng·h/mL) MDZ (day –1) MDZ + BOC (day 6) MDZ (day 8) MDZ (day 13) 9.96 27.6 9.82 8.94 MDZ (day –1) MDZ + BOC (day 6) MDZ (day 8) MDZ (day 13) 52.94 280.7 56.10 43.83 AUC, area under the plasma concentration-time curve; BOC, boceprevir; CI, confidence interval; Cmax, maximum observed plasma concentration; MDZ, midazolam; TID, three times a day. 277 (236–325) 530 (466–603) Drospirenone/Ethinyl estradiol N = 16 healthy volunteers BOC 800 mg TID Oral contraceptive: DRSP 3 mg/EE 0.02 mg QD Day 1 Day 8 Treatment Day 14 LS Meana Ratio Estimate, % (90% CI) Effect of BOC (800 mg TID) on DRSP Cmax (ng/mL) OC OC + BOC 46.0 73.0 157 (146–170) AUC(0-8h) (ng·h/mL) OC OC + BOC 655 1304 199 (187–211) Effect of BOC (800 mg TID) on EE Cmax (ng/mL) OC OC + BOC 54.0 54.0 100 (91–110) AUC(0-24h) (ng·h/mL) OC OC + BOC 659 499 76 (73–79) aModel-based (least squares) geometric mean; ANOVA extracting the effects due to treatment and subject. AUC, area under the plasma concentration-time curve; BOC, boceprevir; CI, confidence interval; Cmax, maximum observed plasma concentration; DRSP, drospirenone; EE, ethinylestradiol; LS, least squares; OC, oral contraceptive; QD, once daily; TID, three times a day. Drug-Drug Interactions: Boceprevir As Victim No Clinically Relevant Effect of Co-administered Drugs on Boceprevir AKR inhibitors CYP3A4/P-gp inhibitors CYP3A4 inducers Other † Co-administered Drug Mean AUC( ) Ratio† Ibuprofen Diflunisal Ketoconazole Ritonavir Clarithromycin* Efavirenz Tenofovir Peginterferon -2b Ribavirin 1.04 ↔ 0.96 ↔ 2.31 ↑ 0.81 ↔ 1.21 ↔ 0.81 ↔ 1.08 ↔ 1.00 ↔ ~0.92 ↔ Ratio estimate of boceprevir PK parameters (in combination vs. alone); = ratio estimate <0.8; = ratio estimate ≥0.8 and ≤1.25; = ratio estimate >1.25. * in presence of diflunisal, compared with boceprevir + diflunisal Drug-Drug Interactions: Boceprevir As Perpetrator Effect of Boceprevir on Co-administered Drugs is Predictable Co-administered Drug CYP3A4 substrates Other Midazolam Drospirenone/ Ethinyl estradiol Efavirenz Tenofovir Peginterferon -2b Ribavirin‡ Mean AUC( ) Ratio† 5.30 ↑ 1.99 ↑ 0.76 ↓ 1.20 ↔ 1.05 ↔ 0.99 ↔ ~0.98 ↔ Phase 3 sub-analyses: similar safety profile when CYP3A4 substrates (eg. benzodiazepines) or inhibitors (eg. azoles) administered with boceprevir † Ratio estimate of concomitant drug PK parameters (in combination with Boceprevir vs. alone); = ratio estimate <0.8; = ratio estimate ≥0.8 and ≤1.25; = ratio estimate >1.25. No Correlation of SVR With Plasma PK No SVR (n=29) SVR (n=87) No SVR (n=29) SVR (n=87) Tx-Experienced Tx-Naive Median, Quartiles Data from RESPOND-2 and SPRINT-2. AUC=area under the concentration-time curve; Cmin=minimum observed plasma concentration; PK=pharmacokinetic; SVR=sustained virologic response. Drug-Drug Interactions: Summary & Conclusions Unlikely Victim • Metabolized by two pathways o Clinically relevant AKR inhibitors not known o Effect of CYP3A4 inhibitors & inducers modest o Drugs affecting other enzymes/transporters unlikely to alter boceprevir o No dose adjustments of boceprevir required Predictable Perpetrator • Interaction with sensitive CYP3A4 substrate drugs should be expected • Many drugs are CYP3A4 inhibitors o These interactions are understood and managed appropriately o CYP3A4 substrate drugs with toxicities often dose-titrated as standard practice o Many drug classes include alternatives that are not CYP3A4 substrates o No interaction with drugs metabolized by other pathways, such as standard of care (Peginterferon -2b / ribavirin) Thanks to: • the subjects and their families who participated in these studies • investigators • Merck colleagues