Ruff_Meta-analysis - Clinical Trial Results
advertisement

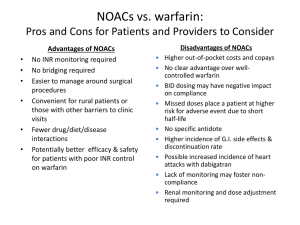
Comprehensive Meta-Analysis Comparing the Efficacy and Safety of NOACs with Warfarin in AF An Analysis Including 71,683 Patients from Four Large Randomized Clinical Trials Christian T. Ruff, MD, MPH TIMI Study Group Brigham and Women’s Hospital Harvard Medical School Boston, MA 1 Pivotal Warfarin-Controlled Trials Stroke Prevention in AF Warfarin vs. Placebo 2,900 Patients NOACs vs. Warfarin 71,683 Patients ROCKET AF (Rivaroxaban) 2010 6 Trial of Warfarin vs. Placebo 1989-1993 RE-LY (Dabigatran) 2009 ENGAGE AF-TIMI 48 (Edoxaban) 2013 ARISTOTLE (Apixaban) 2011 2 Comparative PK/PD of NOACs Dabigatran Rivaroxaban Apixaban Edoxaban IIa (thrombin) Xa Xa Xa Hours to Cmax 1-3 2-4 3-4 1-2 Half-life, hours 12-17 5-13 12 10-14 80 33* 27 50 Transporters P-gp P-gp P-gp P-gp CYP Metabolism, % None 32 <32 <4 Target Renal Clearance, % CYP = cytochrome P450; P-gp = P-glycoprotein *33% renally cleared; 33% excreted unchanged in urine Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc. 2013 Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc. 2011 Weinz et al. Drug Dispos Metab 2009;37:1056–1064 ELIQUIS Summary of Product Characteristics. Bristol Myers Squibb/Pfizer EEIG, UK Matsushima et al. Am Assoc Pharm Sci 2011; abstract Ogata, et al. J Clin Pharmacol 2010;50:743–753 Mendell, et al. Am J Cardiovasc Drugs 2013;13:331–342 Bathala, et al. Drug Metab Dispos 2012;40:2250–2255 3 NOAC SPAF Trials Drug # Randomized Dose (mg) Frequency Dose Adjustment At Baseline After Randomization Target INR (Warfarin) Design RE-LY ROCKET-AF ARISTOTLE ENGAGE AF Dabigatran Rivaroxaban Apixaban Edoxaban 18,113 14,266 18,201 21,105 150, 110 20 5 60, 30 Twice Daily Once Daily Twice Daily Once Daily No 20 → 15 5 → 2.5 60 → 30 30 → 15 0 21 5 25 No No No >9% 2.0-3.0 2.0-3.0 2.0-3.0 2.0-3.0 PROBE* 2x blind 2x blind 2x blind *PROBE = prospective, randomized, open-label, blinded end point evaluation Connolly SJ, et al. N Engl J Med 2009;361:1139-1151 Patel MR, et al. N Engl J Med 2011;365:883-891 Granger CB, et al. N Engl J Med 2011;365:981-992 Giugliano RP, et al. N Engl J Med 2013; e-pub ahead of print DOI:10.1056/NEJMoa1310907 4 Baseline Characteristics RE-LY (Dabigatran) ROCKET-AF (Rivaroxaban) ARISTOTLE (Apixaban) ENGAGE AF (Edoxaban) # Randomized 18,113 14,264 18,201 21,105 Age, years 72 ± 9 73 [65-78] 70 [63-76] 72 [64-78] Female, % 37 40 35 38 Paroxysmal AF 32 18 15 25 VKA naive 50 38 43 41 Aspirin Use 40 36 31 29 CHADS2 0-1 2 3-6 13 33 32 35 87 30 34 53 47 36 Connolly SJ, et al. N Engl J Med 2009;361:1139-1151 Patel MR, et al. N Engl J Med 2011;365:883-891 Granger CB, et al. N Engl J Med 2011;365:981-992 Giugliano RP, et al. N Engl J Med 2013; e-pub ahead of print DOI:10.1056/NEJMoa1310907 5 Trial Metrics RE-LY (Dabigatran) ROCKET-AF (Rivaroxaban) ARISTOTLE (Apixaban) ENGAGE AF (Edoxaban) Median Follow-Up, years 2.0 1.9 1.8 2.8 Median TTR 66 58 66 68 Lost to Follow-Up, N 20 32 90 1 *TTR, time in therapeutic range Connolly SJ, et al. N Engl J Med 2009;361:1139-1151 Patel MR, et al. N Engl J Med 2011;365:883-891 Granger CB, et al. N Engl J Med 2011;365:981-992 Giugliano RP, et al. N Engl J Med 2013; e-pub ahead of print DOI:10.1056/NEJMoa1310907 6 All NOACS: Stroke or SEE Risk Ratio (95% CI) 0.66 (0.53 - 0.82) RE-LY [150 mg] ROCKET AF 0.88 (0.75 - 1.03) ARISTOTLE 0.80 (0.67 - 0.95) ENGAGE AF-TIMI 48 0.88 (0.75 - 1.02) [60 mg] Combined 0.81 (0.73 - 0.91) [Random Effects Model] N=58,541 0.5 Heterogeneity p=0.13 p=<0.0001 Favors NOAC 1 Favors Warfarin 2 Ruff CT, et al. Lancet 2013 [in-press] 7 Secondary Efficacy Outcomes Risk Ratio (95% CI) Ischemic Stroke 0.92 (0.83 - 1.02) p=0.10 Hemorrhagic Stroke 0.49 (0.38 - 0.64) p<0.0001 MI 0.97 (0.78 - 1.20) p=0.77 All-Cause Mortality 0.90 (0.85 - 0.95) p=0.0003 0.2 0.5 Favors NOAC 1 2 Favors Warfarin Heterogeneity p=NS for all outcomes Ruff CT, et al. Lancet 2013 [in-press] 8 All NOACS: Major Bleeding Risk Ratio (95% CI) 0.94 (0.82 - 1.07) RE-LY [150 mg] ROCKET AF 1.03 (0.90 - 1.18) ARISTOTLE 0.71 (0.61 - 0.81) ENGAGE AF-TIMI 48 0.80 (0.71 - 0.90) [60 mg] Combined 0.86 (0.73 - 1.00) [Random Effects Model] p=0.06 N=58,498 0.5 Heterogeneity p=0.001 Favors NOAC 1 Favors Warfarin 2 Ruff CT, et al. Lancet 2013 [in-press] 9 Secondary Safety Outcomes Risk Ratio (95% CI) 0.48 (0.39 - 0.59) ICH p<0.0001 1.25 (1.01 - 1.55) GI Bleeding 0.2 p=0.043 0.5 Favors NOAC 1 2 Favors Warfarin Heterogeneity ICH, p=0.22 GI Bleeding, p=0.009 Ruff CT, et al. Lancet 2013 [in-press] 10 Subgroups: Stroke or SEE Age Gender Diabetes Prior Stroke or TIA CrCl CHADS2 Score VKA Status Center-Based TTR Risk Ratio (95% CI) P-Interaction <75 0.85 (0.73 - 0.99) p=0.38 ≥75 0.78 (0.68 - 0.88) Female 0.78 (0.65 - 0.94) Male 0.84 (0.75 - 0.94) No 0.83 (0.74 - 0.93) Yes 0.80 (0.69 - 0.93) No 0.78 (0.66 - 0.91) Yes 0.86 (0.76 - 0.98) <50 0.79 (0.65 - 0.96) 50-80 0.75 (0.66 - 0.85) >80 0.98 (0.79 - 1.22) 0-1 0.75 (0.54 - 1.04) 2 0.86 (0.70 - 1.05) 3-6 0.80 (0.72 - 0.89) Naive 0.75 (0.66 - 0.86) Experienced 0.85 (0.70 - 1.03) <66% 0.77 (0.65 - 0.92) ≥66% 0.82 (0.71 - 0.95) 0.5 Ruff CT, et al. Lancet 2013 [in-press] 1 Favors NOAC p=0.52 p=0.73 p=0.30 p=0.12 p=0.76 p=0.31 p=0.60 2 Favors Warfarin 11 Subgroups: Major Bleeding Age Gender Diabetes Prior Stroke or TIA CrCl CHADS2 Score VKA Status Center-Based TTR Risk Ratio (95% CI) P-Interaction <75 0.79 (0.67 - 0.94) p=0.28 ≥75 0.93 (0.74 - 1.17) Female 0.75 (0.58 - 0.97) Male 0.90 (0.72 - 1.12) No 0.71 (0.54 – 0.93) Yes 0.90 (0.78 - 1.04) No 0.85 (0.72 - 1.01) Yes 0.89 (0.77 - 1.02) <50 0.74 (0.52 - 1.05) 50-80 0.91 (0.76 - 1.08) >80 0.85 (0.66 - 1.10) 0-1 0.60 (0.45 - 0.80) 2 0.88 (0.65 - 1.20) 3-6 0.86 (0.71 - 1.04) Naive 0.84 (0.76 - 0.93) Experienced 0.87 (0.70 - 1.08) <66% 0.69 (0.59 - 0.81) ≥66% 0.93 (0.76 - 1.13) 0.2 Ruff CT, et al. Lancet 2013 [in-press] 0.5 Favors NOAC 1 2 Favors Warfarin p=0.29 p=0.12 p=0.70 p=0.57 p=0.09 p=0.78 p=0.022 12 Low Dose Regimens Efficacy & Safety Outcomes Dabigatran 110 mg & Edoxaban 30 mg Risk Ratio (95% CI) 1.03 (0.84 - 1.27) Stroke or SEE p=0.74 1.28 (1.02 - 1.60) Ischemic Stroke p=0.045 0.33 (0.23 - 0.46) Hemorrhagic Stroke p<0.0001 MI 1.25 (1.04 - 1.50) p=0.019 0.89 (0.83 - 0.96) All-Cause Mortality p=0.003 0.65 (0.43 - 1.00) Major Bleeding p=0.05 0.31 (0.24 - 0.41) ICH p<0.0001 0.89 (0.57 - 1.37) GI Bleeding p=0.58 N=26,107 Heterogeneity P=NS for outcomes except: Major Bleeding, p=<0.001 GI Bleeding, p=0.01 0.2 0.5 Favors Low Dose NOAC 1 2 Favors Warfarin Ruff CT, et al. Lancet 2013 [in-press] 13 Comprehensive Meta-Analysis Comparing the Efficacy and Safety of NOACs with Warfarin in AF An Analysis Including 71,683 Patients from Four Large Randomized Clinical Trials Christian T. Ruff, MD, MPH TIMI Study Group Brigham and Women’s Hospital Harvard Medical School Boston, MA 14 Disclosures Research Support: Daiichi Sankyo, AstraZeneca Consultant and Advisory Boards: Boehringer Ingelheim, Daiichi Sankyo, BristolMeyers Squibb 15 Stroke Prevention in AF Warfarin vs. Placebo AFASAK-1 (671) SPAF (421) BAATAF (420) CAFA (378) SPINAF (571) EAFT (439) All Trials (n=6) 64% 100% 50% Warfarin Better 0% -50% -100% Warfarin Worse Hart RG, et al. Ann Intern Med 2007;146:857-867. 16 Pivotal Warfarin-Controlled Trials Stroke Prevention in AF Warfarin vs. Placebo 2,900 Patients NOACs vs. Warfarin 71,683 Patients ROCKET AF (Rivaroxaban) 2010 6 Trials of Warfarin vs. Placebo 1989-1993 RE-LY (Dabigatran) 2009 ENGAGE AF-TIMI 48 (Edoxaban) 2013 ARISTOTLE (Apixaban) 2011 17 Meta-Analysis First to contain data from all 4 phase 3 warfarin-controlled trials Robust sample size − − Precision in assessing relative benefit of NOACs in key clinical subgroups Effects of agents on important secondary outcomes Pooled data for FXa and thrombin inhibitors − − − Target key coagulation enzymes Trials share similar design Agents used interchangeably clinically and grouped together by Guidelines Separate meta-analysis of low dose dabigatran and edoxaban Comprehensive picture of the NOACs as a therapeutic option 18 Comparative PK/PD of NOACs Dabigatran Rivaroxaban Apixaban Edoxaban IIa (thrombin) Xa Xa Xa Hours to Cmax 1-3 2-4 3-4 1-2 Half-life, hours 12-17 5-13 12 10-14 80 33* 27 50 Transporters P-gp P-gp P-gp P-gp CYP Metabolism, % None 32 <32 <4 Target Renal Clearance, % CYP = cytochrome P450; P-gp = P-glycoprotein *33% renally cleared; 33% excreted unchanged in urine Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc. 2013 Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc. 2011 Weinz et al. Drug Dispos Metab 2009;37:1056–1064 ELIQUIS Summary of Product Characteristics. Bristol Myers Squibb/Pfizer EEIG, UK Matsushima et al. Am Assoc Pharm Sci 2011; abstract Ogata, et al. J Clin Pharmacol 2010;50:743–753 Mendell, et al. Am J Cardiovasc Drugs 2013;13:331–342 Bathala, et al. Drug Metab Dispos 2012;40:2250–2255 19 NOAC SPAF Trials Drug # Randomized Dose (mg) Frequency Dose Adjustment At Baseline After Randomization Target INR (Warfarin) Design RE-LY ROCKET-AF ARISTOTLE ENGAGE AF Dabigatran Rivaroxaban Apixaban Edoxaban 18,113 14,266 18,201 21,105 150, 110 20 5 60, 30 Twice Daily Once Daily Twice Daily Once Daily No 20 → 15 5 → 2.5 60 → 30 30 → 15 0 21 5 25 No No No >9% 2.0-3.0 2.0-3.0 2.0-3.0 2.0-3.0 PROBE* 2x blind 2x blind 2x blind *PROBE = prospective, randomized, open-label, blinded end point evaluation Connolly SJ, et al. N Engl J Med 2009;361:1139-1151 Patel MR, et al. N Engl J Med 2011;365:883-891 Granger CB, et al. N Engl J Med 2011;365:981-992 Giugliano RP, et al. N Engl J Med 2013; e-pub ahead of print DOI:10.1056/NEJMoa1310907 20 Baseline Characteristics RE-LY (Dabigatran) ROCKET-AF (Rivaroxaban) ARISTOTLE (Apixaban) ENGAGE AF (Edoxaban) # Randomized 18,113 14,264 18,201 21,105 Age, years 72 ± 9 73 [65-78] 70 [63-76] 72 [64-78] Female, % 37 40 35 38 Paroxysmal AF 32 18 15 25 VKA naive 50 38 43 41 Aspirin Use 40 36 31 29 CHADS2 0-1 2 3-6 13 33 32 35 87 30 34 53 47 36 Connolly SJ, et al. N Engl J Med 2009;361:1139-1151 Patel MR, et al. N Engl J Med 2011;365:883-891 Granger CB, et al. N Engl J Med 2011;365:981-992 Giugliano RP, et al. N Engl J Med 2013; e-pub ahead of print DOI:10.1056/NEJMoa1310907 21 Trial Metrics RE-LY (Dabigatran) ROCKET-AF (Rivaroxaban) ARISTOTLE (Apixaban) ENGAGE AF (Edoxaban) Median Follow-Up, years 2.0 1.9 1.8 2.8 Median TTR 66 58 66 68 Lost to Follow-Up, N 20 32 90 1 *TTR, time in therapeutic range Connolly SJ, et al. N Engl J Med 2009;361:1139-1151 Patel MR, et al. N Engl J Med 2011;365:883-891 Granger CB, et al. N Engl J Med 2011;365:981-992 Giugliano RP, et al. N Engl J Med 2013; e-pub ahead of print DOI:10.1056/NEJMoa1310907 22 All NOACS: Stroke or SEE Risk Ratio (95% CI) 0.66 (0.53 - 0.82) RE-LY [150 mg] ROCKET AF 0.88 (0.75 - 1.03) ARISTOTLE 0.80 (0.67 - 0.95) ENGAGE AF-TIMI 48 0.88 (0.75 - 1.02) [60 mg] Combined 0.81 (0.73 - 0.91) [Random Effects Model] N=58,541 0.5 Heterogeneity p=0.13 p=<0.0001 Favors NOAC 1 Favors Warfarin 2 Ruff CT, et al. Lancet 2013 [in-press] 23 Secondary Efficacy Outcomes Risk Ratio (95% CI) Ischemic Stroke 0.92 (0.83 - 1.02) p=0.10 Hemorrhagic Stroke 0.49 (0.38 - 0.64) p<0.0001 MI 0.97 (0.78 - 1.20) p=0.77 All-Cause Mortality 0.90 (0.85 - 0.95) p=0.0003 0.2 0.5 Favors NOAC 1 2 Favors Warfarin Heterogeneity p=NS for all outcomes Ruff CT, et al. Lancet 2013 [in-press] 24 All NOACS: Major Bleeding Risk Ratio (95% CI) 0.94 (0.82 - 1.07) RE-LY [150 mg] ROCKET AF 1.03 (0.90 - 1.18) ARISTOTLE 0.71 (0.61 - 0.81) ENGAGE AF-TIMI 48 0.80 (0.71 - 0.90) [60 mg] Combined 0.86 (0.73 - 1.00) [Random Effects Model] p=0.06 N=58,498 0.5 Heterogeneity p=0.001 Favors NOAC 1 Favors Warfarin 2 Ruff CT, et al. Lancet 2013 [in-press] 25 Secondary Safety Outcomes Risk Ratio (95% CI) 0.48 (0.39 - 0.59) ICH p<0.0001 1.25 (1.01 - 1.55) GI Bleeding 0.2 p=0.043 0.5 Favors NOAC 1 2 Favors Warfarin Heterogeneity ICH, p=0.22 GI Bleeding, p=0.009 Ruff CT, et al. Lancet 2013 [in-press] 26 Subgroups: Stroke or SEE Age Gender Diabetes Prior Stroke or TIA CrCl CHADS2 Score VKA Status Center-Based TTR Risk Ratio (95% CI) P-Interaction <75 0.85 (0.73 - 0.99) p=0.38 ≥75 0.78 (0.68 - 0.88) Female 0.78 (0.65 - 0.94) Male 0.84 (0.75 - 0.94) No 0.83 (0.74 - 0.93) Yes 0.80 (0.69 - 0.93) No 0.78 (0.66 - 0.91) Yes 0.86 (0.76 - 0.98) <50 0.79 (0.65 - 0.96) 50-80 0.75 (0.66 - 0.85) >80 0.98 (0.79 - 1.22) 0-1 0.75 (0.54 - 1.04) 2 0.86 (0.70 - 1.05) 3-6 0.80 (0.72 - 0.89) Naive 0.75 (0.66 - 0.86) Experienced 0.85 (0.70 - 1.03) <66% 0.77 (0.65 - 0.92) ≥66% 0.82 (0.71 - 0.95) 0.5 Ruff CT, et al. Lancet 2013 [in-press] 1 Favors NOAC p=0.52 p=0.73 p=0.30 p=0.12 p=0.76 p=0.31 p=0.60 2 Favors Warfarin 27 Subgroups: Major Bleeding Age Gender Diabetes Prior Stroke or TIA CrCl CHADS2 Score VKA Status Center-Based TTR Risk Ratio (95% CI) P-Interaction <75 0.79 (0.67 - 0.94) p=0.28 ≥75 0.93 (0.74 - 1.17) Female 0.75 (0.58 - 0.97) Male 0.90 (0.72 - 1.12) No 0.71 (0.54 – 0.93) Yes 0.90 (0.78 - 1.04) No 0.85 (0.72 - 1.01) Yes 0.89 (0.77 - 1.02) <50 0.74 (0.52 - 1.05) 50-80 0.91 (0.76 - 1.08) >80 0.85 (0.66 - 1.10) 0-1 0.60 (0.45 - 0.80) 2 0.88 (0.65 - 1.20) 3-6 0.86 (0.71 - 1.04) Naive 0.84 (0.76 - 0.93) Experienced 0.87 (0.70 - 1.08) <66% 0.69 (0.59 - 0.81) ≥66% 0.93 (0.76 - 1.13) 0.2 Ruff CT, et al. Lancet 2013 [in-press] 0.5 Favors NOAC 1 2 Favors Warfarin p=0.29 p=0.12 p=0.70 p=0.57 p=0.09 p=0.78 p=0.022 28 ACTIVE-W: Stroke or SEE TTR ≥ 65% TTR < 65% 0 .1 0 RR = 1.83 RR = 1.11 P = 0.47 0 .0 8 P < 0.0001 0 .0 6 0 .0 4 Clopi + ASA C+A OAC VKA 0 .0 2 0 .0 4 0 .0 6 Clopi + ASA C+A 0 .0 2 Event Rate (%) 0 .0 8 0 .1 0 P-interaction = 0.013 OAC 0 .0 0 .0 VKA 0.0 0.5 1.0 Years Connolly SJ, et al. Circulation 2008;118:2029-2037 1.5 0.0 0.5 1.0 1.5 Years 29 ACTIVE-W: Major Bleeding TTR ≥ 65% TTR < 65% RR = 1.55 RR = 0.68 0 .0 4 P = 0.08 0 .0 3 0 .0 3 0 .0 4 P = 0.027 OAC 0 .0 2 0 .0 2 C+A C+A 0 .0 0 .0 1 0 .0 1 OAC 0 .0 Event Rate (%) 0 .0 5 0 .0 5 P-interaction = 0.0006 0.0 0.5 1.0 Years Connolly SJ, et al. Circulation 2008;118:2029-2037 1.5 0.0 0.5 1.0 1.5 Years 30 Low Dose Regimens Efficacy & Safety Outcomes Dabigatran 110 mg & Edoxaban 30 mg Risk Ratio (95% CI) 1.03 (0.84 - 1.27) Stroke or SEE p=0.74 1.28 (1.02 - 1.60) Ischemic Stroke p=0.045 0.33 (0.23 - 0.46) Hemorrhagic Stroke p<0.0001 MI 1.25 (1.04 - 1.50) p=0.019 0.89 (0.83 - 0.96) All-Cause Mortality p=0.003 0.65 (0.43 - 1.00) Major Bleeding p=0.05 0.31 (0.24 - 0.41) ICH p<0.0001 0.89 (0.57 - 1.37) GI Bleeding p=0.58 N=26,107 Heterogeneity P=NS for outcomes except: Major Bleeding, p=<0.001 GI Bleeding, p=0.01 0.2 0.5 Favors Low Dose NOAC 1 2 Favors Warfarin Ruff CT, et al. Lancet 2013 [in-press] 31 Conclusions NOACs significantly reduce stroke (19%) − Primarily driven by reduction in hemorrhagic stroke (51%) NOACs significantly reduce mortality (10%) Trend toward less bleeding − − Substantial reduction in ICH (52%) Increased GI bleeding (25%) The relative efficacy and safety of NOACs consistent across a wide spectrum of AF patients − Even less bleeding when INR not as well controlled Low dose NOAC regimens reduce mortality and have a very favorable bleeding profile but more ischemic events Differences in agents, patients, and trials may not be accounted for − Heterogeneity major bleeding and GI bleeding 32 BACK – UP 33 Factor Xa Inhibitors: Stroke or SEE Risk Ratio (95% CI) ROCKET AF 0.88 (0.75 - 1.03) ARISTOTLE 0.80 (0.67 - 0.95) ENGAGE AF-TIMI 48 0.88 (0.75 - 1.02) Combined 0.86 (0.78 - 0.94) [Random Effects Model] p=0.0011 N=46,443 0.5 1 Favors NOAC Heterogeneity p=0.65 2 Favors Warfarin 34 Factor Xa Inhibitors: Bleeding Risk Ratio (95% CI) 1.03 (0.89, 1.18) ROCKET 0.70 (0.61, 0.81) ARISTOTLE 0.80 (0.71, 0.90) ENGAGE AF-TIMI 48 0.83 (0.68, 1.02) Combined [Random Effects Model] N=46,400 Favors NOAC Heterogeneity p=0.0006 2 1 0.5 Favors Warfarin 35