Chapter 3 PPT
advertisement

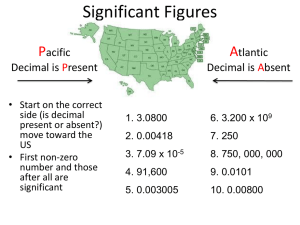
Chapter 3 Scientific Measurement You WILL be able to… Convert measurements to Scientific notation Distinguish between accuracy, precision, and error of a measurement. List SI units of measurement and common Prefixes Distinguish between mass and weight of an object Convert between Celsius and Kelvin Construct conversion factors Apply dimensional analysis Convert complex units Calculate density of a material Describe how density varies with temp. Le Systeme International d’Unites (SI) Also known as the METRIC system Developed in France in 1795 Based on multiples of 10 Standard of measurements used in the scientific community and industry in order to speak the “same language” Why is it important? To compare results To communicate effectively 5 SI units commonly used by chemists are the meter, the kilogram, the Kelvin, the second, and the mole. SI Unit Prefixes See table on page 74. Do you recognize any of the Prefixes? Units and Quantities Units of Length - measure of distances, standard is the meter. This is a FUNDAMENTAL PHYSICAL QUANTITY Units of Volume – the amount of space occupied by an object (substance), standard is the CUBIC METER (m3 ). This is a DERIVED unit Units of Mass – of the quantity of matter, represented by the KILOGRAM Weight - measure of the gravitational pull on matter and is NOT an SI base unit! (e.g. scale activity) b. standard is the kilogram (why??) Units and Quantities cont’d Units of Temperature – measure of average kinetic energy of particles of matter based on the triple point of water, standard is the Kelvin a. This is a FUNDAMENTAL unit b. The Celsius Scale is commonly used in chemistry c. To convert Celsius to Kelvin, add 273.15 Units of Energy – energy is the capacity to do work or to produce heat a. this is a DERIVED unit b. standard is the Joule (J), but calorie is commonly used c. 1 cal = 4.184J Try These! List the following units in order from largest to smallest: m3, mL, cL, μL, L, dL -You should have come up with … m3 , L, dL, cL, mL, μL Which would melt first, germanium with a melting point of 1210K or gold with a melting point of 1064 ˚C? A MEASUREMENT is a quantity that has both a number (magnitude) and a UNIT In chemistry we will deal with extremely large and small numbers. We will use scientific notation to deal with these cumbersome numbers. Scientific Notation One number will be represented as the product of two numbers. For example: N x 10y y is an integer which shows how far to move the decimal. If the number is greater than one y is POSITIVE, move the decimal to the right to expand. If the number is less than one y is NEGATIVE, move the decimal to the left to expand. Try These Write these in Scientific Notation 602000000000000000000000 .000000000000000000000327 Expand the following 3.0 x 108 4.2 x 10-5 How do you enter this into your calculator? Mathematical Operations Using Scientific Notation Addition and Subtraction – can be done ONLY if the values have the same exponent (n) This means that you may have to change one of the values out of proper scientific notation to perform the calculation For example: a. (5.3 x 104) + (1.3 x 103) b. (5.3 x 104) - (1.3 x 103) a. b. = 5.43x104 = 5.17x104 Multiplying and Dividing Multiplication and Division – can be done with any values. When you MULTIPLY you will ADD the exponents. When you DIVIDE you will SUBTRACT the exponents. For Example: a. (7.2 x 10-4) ÷ (1.8 x 103) = b. (7.2 x 10-4) x (1.8 x 103) = A = 4 x 10-7 B = 1.3 Accuracy, Precision, and Error Accuracy – a measure of how close a measurement comes to the actual or true value of whatever is measured (correctness of measurements) Precision – a measure of how close a series of measurements are to one another (repeatability of measurements) Precision and Accuracy Determining Error ERROR is the difference between the experimental value and the accepted value Error = Experimental Value – Accepted Value Percent Error takes this difference and divides it by the accepted value (times 100) to get a relative error Experimentally you determined the boiling point of octane to be 124.1˚C. The actual boiling point of octane is 125.7˚C. Calculate the error and the percent error. Use Percent Error = │error│ X 100 accepted value This will be used all year LEARN IT! Significant Figures in Measurements What is a Sig Fig? All the digits in a measurement plus one ESTIMATED digit. Rule # 1 – All non-zero digits are significant (23.45) Rule # 2 – All zeroes between non zero digits are significant (sandwich rule) (304) Rule # 3 – Zeroes to the right of a nonzero digit, but to the left of an unwritten decimal point, are NOT significant. (5000) or (5000.) Rule # 4 - In numbers less than one, zeros to the right of a decimal point and to the left of a non-zero digit are NOT significant. (.0034) Rule # 5 All zeros to the right of a decimal point AND to the right of a nonzero digit ARE significant (.8400) TAKE A DEEP BREATH! Decimal point is PRESENT 0.00026050 mg Find the first digit & count until the end of the number Decimal point is ABSENT 870 000 g Find the first digit & count until the beginning of the number Apply the Rules to the Following How many Sig Figs? 1. 2. 3. 4. 5. 1. 2. 3. 4. 5. 7.36 cm 85 000 g 29.40 ml 0.00183 s 6 000 000 ˚C 3 2 4 3 1 Math with Sig Figs General Rule – A calculated answer cannot be more PRECISE than the LEAST precise measurement from which it was calculated Addition and Subtraction – The answer should be rounded to the same number of DECIMAL PLACES (not digits) as the measurement with the least number of decimals places Multiplication and Division - When multiplying and dividing, the answer can have no more SIG FIGS than are in the measurement with the FEWEST number of Sig. Figs ROUNDING Rounding – Simplified version is that 5’s round up. Apply all the rules to the following… 1. 3.29 cm – 1.4 cm = 2. 80500 km X 0.0060 km = 3. Round 629.55 meters to 3 Sig. Figs 1= 1.9 cm 2= 480 km2 3= 630. (note the decimal) Dimensional Analysis/FactorLabel Method Dimensional Analysis is treating units of measure as algebraic terms. The factor label method allows you to convert from one unit to another -In order to change units you need a CONVERSION FACTOR. A conversion factor is a ratio derived from the equality between two different units that can be used to convert from one unit to the other or a ratio of equivalent measurements. Conversion factors are known, verified, exact quantities therefore, they are NOT used to determine Sig. Figs in a problem Sig. Figs in a problem are determined by the original measurements/ data given Converting Between Units Rule – The smaller number has the larger unit and the larger number has the smaller unit in a conversion factor (e.g. 1 km = 1000 m) Multi-step Problems – You may need to use several conversion factors to get from the unit that you have to the unit that you need. Do this by memorizing the common conversions only, rather than memorizing every possible metric conversion. Sample Problem – The radius of a potassium atom is 0.227nm. Express this radius unit in centimeters. If you remember that “nano” is 10-9 and “centi” is 10-2 you can subtract exponents and do this in one step. However, if you need to see the steps written out, do the following: 0.227nm X 1m X 1 000 000 000 nm 100 cm 1m = Converting Complex Units – This applies to most derived units which are a combination of quantities. Both the numerator and the denominator must be changed, so more than on step is necessary. Try This One! Gold has a density of 19.3 g/cm3. What is the density in kilograms per cubic meter? Answer… 1.93 x 10-4 kg/m3 Density Density is the ratio of mass to volume of a substance The standard is the kg/m3 Equation: D = m/V Bunsen Honeydew finds a shiny piece of metal that he thinks is aluminum. In the lab, he determines that the metal has a volume of 245.0 cm3 (how did he do that?) and a mass of 612.00 g. Calculate the density in correct Sig. Figs. Is the metal aluminum? Density and Temperature The density of MOST substances decreases as temperature increases. List some examples of this from common experience. Water is an exception which allows for life on Earth to be possible