L03B - Clarkson University
advertisement

L03B: Chapter 3 (continued)
• Note that an understanding of crystal structure is essential for doing well in
the rest of this course.
• So you should be reading the text and doing example problems.
• Review the lectures and make certain you understand everything.
• If you don't, ask questions by email (wilcox@clarkson.edu).
• In this lecture we cover the following:
− Closed-packed metal structures: Face-centered cubic and hexagonal close
packed.
− Methods to denote directions and planes in hexagonal structures.
VERY DIFFERENT !
− Polymorphism in carbon: diamond, graphite, graphene, buckeyballs, nanofibers, amorphous, etc.
W.R. Wilcox, Clarkson University. Last revised September 12, 2013
FCC Stacking Sequence
ABCABC... Stacking sequence of {111} close-packed planes.
B
A
A sites
B sites
B
C
B
B
C
B
C
B
B
C sites
A
B
C
2
Hexagonal Close-Packed Structure (HCP)
ABAB... Stacking Sequence for close-packed planes in HCP
Top
A sites
layer
Middle layer
Bottom layer
c
B sites
A sites
a
Hexagonal unit cell
examples: Cd, Mg, Ti, Zn
6 atoms/unit cell
c/a = 1.633
APF = 0.74
The only difference between FCC and HCP is second-nearest neighbors.
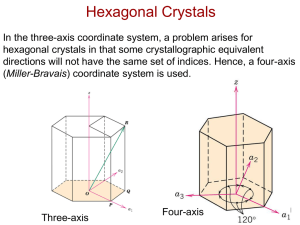
Crystallographic Directions in a Hexagonal Structure
• Miller-Bravais lattice
• 4 axes: a1, a2, a3, z
• Dimensions are a (for a1, a2,
and a3 axes) and c (for z-axis)
• Direction [uvtw]
• Algorithm to draw vector.
– Remove brackets
– Divide by largest integer so all
values are ≤ 1
– Multiply terms by appropriate unit
cell dimension (a or c) to produce
projections.
– Construct vector by stepping off
these projections.
Example of Drawing a Direction in a Hexagonal Lattice
Draw the [1 2 13] direction in a hexagonal unit cell.
s
Algorithm
a1
a2
a3
z
1. Remove brackets
-1
-2
1
3
2
3
1
3
1
2. Divide by 3
[1213]
-
1
3
-
3. Projections
4. Construct Vector
p
r
q
start at point o
proceed –a/3 units along a1 axis to point p
–2a/3 units parallel to a2 axis to point q
a/3 units parallel to a3 axis to point r
c units parallel to z axis to point s
[1213] direction represented by vector from point o to point s
5
Determination of Miller-Bravais Indices for Direction
Algorithm
1. Vector repositioned (if necessary) to pass
through origin.
2. Read off projections in terms of threeaxis (a1, a2, and z) unit cell dimensions
a and c
3. Adjust to smallest integer values
4. Enclose in square brackets, no commas,
for three-axis coordinates
5. Convert to four-axis Miller-Bravais lattice
coordinates using equations below:
1
u = (2u¢ - v ¢)
3
t = -(u +v)
1
v = (2v ¢ - u¢)
3
w = w¢
6. Adjust to smallest integer values and
enclose in brackets [uvtw]
Example Determination of Indices for Direction
Determine indices for green vector
Example
1. Reposition
2. Projections
3.
Reduction
4.
Brackets
a1
a2
z
not needed
a
a
0c
1
1
0
1
1
0
[110]
5.
6.
Convert to 4-axis parameters
1
1
1
1
u = [(2)(1) - (1)] =
v = [(2)(1) - (1)] =
3
3
3
3
1 1
2
w =0
t = -( + ) = 3 3
3
Reduction & Brackets
1/3, 1/3, -2/3, 0
=>
1, 1, -2, 0
=>
[ 1120 ]
7
Denoting Crystallographic Planes in a Hexagonal Lattice
z
example
1. Intercepts
2. Reciprocals
3.
Reduction
a1
1
1
1
1
a2
1/
0
0
a3
-1
-1
-1
-1
c
1
1
1
1
a2
a3
a1
4.
Miller-Bravais Indices
(1011)
8
Names of planes
• Three names are commonly used for crystallographic planes in the
hexagonal system: basal, prismatic and pyramidal.
• For example, in ice:
• The basal plane is [0001].
• Three prismatic planes are [1000], [0100] and
[0010].
• The pyramidal planes intersect the c axis at an
angle. Example of a hexagonal pyramid:
Polymorphic Forms of Carbon
Diamond
VMSE
• Very strong covalent tetrahedral
bonding.
• Consequently, very few free electrons
and so is an electrical insulator.
• Single crystal diamond has many
exceptional properties, e.g.:
– Hardest material
– Highest thermal conductivity
• Diamond cubic structure.
• Can also be considered face-centered
cubic, but not close packed.
• Each fcc lattice site has 2 atoms.
• The group IV semiconductors, Si and
Ge, also have the diamond structure.
• Integrated circuits are made from Si.
• Hexagonal diamond (Lonsdaleite)
discovered in meteorites:
http://en.wikipedia.org/wiki/Lonsdaleite
Diamond synthesis
•
•
•
•
Diamond is thermodynamically stable only at high pressure.
Created in the earth at high pressure.
Graphite is the stable structure at atmospheric conditions.
At room temperature, the rate of transformation to graphite is
negligible.
• Crystals, powder and coatings are made synthetically:
• High pressure
• Low pressure by forming H and CH3 with high T or plasma.
•
e.g.:
http://people.clarkson.edu/~lregel/actaastr.pdf
• Many applications for lab-created diamond, e.g. hard coatings and
abrasives.
Graphite
• Layers with hexagonal structures.
• Very strong covalent bonding within
each hexagonal layer.
• Very weak van der Waal’s bonding
between layers.
• Very anisotropic properties.
• Good electrical conductor within layers.
• Easy separation of the layers.
• Comes in various forms, including small
crystals.
• Has many applications. For example,
see https://en.wikipedia.org/wiki/Graphite
•
VMSE
•
•
Hexagonal BN has the same structure, with
alternating B & N atoms:
http://en.wikipedia.org/wiki/Boron_nitride
Polymorphism for elements is called allotropy
Compounds can also show polymorphism.
Graphene
• A very hot two-dimensional material. See, for example,
http://en.wikipedia.org/wiki/Graphene .
• Originally made by pulling adhesive tape from graphite crystals and
dissolving the tape in a solvent.
• Very unusual thermal, mechanical, chemical, and electronic properties.
• Many potential applications have been demonstrated in the lab.
• The material of the future?
Carbon nanotubes
• Consists of a graphene sheet in the form of a seamless cylinder and closed
by a cap on the end. A one-dimensional structure!
• May have a single wall (graphene layer) or multiple wall, and joined in
different ways.
• Also very unusual properties and many potential applications.
• http://en.wikipedia.org/wiki/Carbon_nanotube
Buckminsterfullerene Molecule
• “Buckey balls”
• C60 molecule consisting of 20 hexagons and
12 pentagons, similar to a soccer ball.
• Covalent bonding.
• Unusual chemical properties.
• Possible use for hydrogen storage.
•
•
http://en.wikipedia.org/wiki/Buckminsterfullerene
For 3D view, open the following in Chrome:
http://www.3dchem.com/molecules.asp?ID=217#
• Three forms of amorphous carbon with commercial applications:
• Glassy, or vitreous, carbon:
http://en.wikipedia.org/wiki/Glassy_carbon
• Carbon fibers
http://en.wikipedia.org/wiki/Carbon_(fiber)
• Diamond-like carbon (DLC):
http://en.wikipedia.org/wiki/Diamond-like_carbon