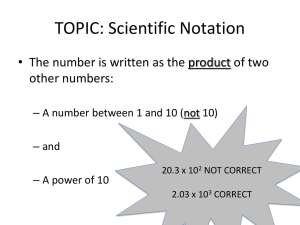
Measuring - Dallas School District
advertisement

Measuring Welcome to Chemistry 1: A college preparatory course. • • • Cellphone = NO!!!! Webpage Need to Buy – Scientific Calculator – Notebook or Binder • Safety Contract – Signed • Course Expectations – – HW & Review (HW Bonus Points) Make up missed work • • – – – – Notes Tests Getting Extra Help Shoes in Locker Letters of recommendation “Are you a student, or just a kid who comes to school?” 1. Three specific things you must do to be successful in this course. 2. Three things you must never do (academically) in this course. Measuring Scientific Method Hypothesis – testable, educated guess Theory - repeatedly confirmed hypothesis that has predictive power. Law – Theory with NO known exceptions Measuring Hypothesis Theory Law Measuring Science changes!!!!! Spotting Bad Science Measuring 1. Based on Anecdotal Evidence Anecdotal - From stories, not studies (no math) 2. Small Sample Size 3. Not published in Journals – not reviewed or tested 4. Broad Claims Example: The Water Cure Measuring Measuring • • Estimated place – every measurement must have ONE estimated place. One place past the smallest marking Measuring Measuring Measuring Measuring Measuring Measuring Measuring Measuring Measuring Measuring Measuring Graphing 1. Label x and y axis including units 2. Mark Axis using a convenient scale 3. Title your graph “The Dependence of Y on X” 4. Mark dots with a small circle 5. Draw “Best Fit” line or curve Measuring Graphing • Best Fit Line a. Used ONLY for linear relationships. b. Fits y = mx + b m = slope b = y-intercept c. If graph is almost perfect line, same # dots above and below X = independent variable (you can control) Y = dependent variable (can’t control) Measuring Measuring Measuring Graphing • Best Fit Curve a. Used if points are clearly not linear. b. Can be fit to higher order eqns: y = mx2 + b Measuring Measuring Graphing Rectangle A = L X W Triangle A = ½ B X H Circle A = r2 Irregular Shape? Measuring Measuring Graphing Lab • • • • Use centimeters TWO decimal places, last one is the estimated place Write down the letter of your shape See me for the actual value Measuring 1. What do chemists study/do? 2. What professions/college majors require a chemistry course? 3. Where is chemistry important in business/industry? 4. What household products are “chemicals”? 5. Where in history was chemistry important? Measuring Scientific Notation 1. Descartes:1637 - “I think, therefore I am” 2. Powers of 10 100 = 1 101 = 10 102 = 10 X 10 = 100 103 = 10 X 10 X 10 =1000 Measuring Scientific Notation 200,000,000,000 stars (Andromeda): 2 X 100,000,000,000 2 X 1011 stars Measuring Scientific Notation 3. A Helium atom masses 0.000,000,000,000,000,000,000,006,645g 6.645 X 10-24 g Measuring Scientific Notation 340 378,400 0.00234 0.000 000 000 0918 5.6 X 105 6.12 X 10-3 2.6 X 10-7 4 x 102 Measuring Scientific Notation 43 575,400 0.000723 0.000 000 0014 6.5 X 10-5 2.16 X 103 6.2 X 107 8 x 10-2 Measuring Scientific Notation There are ~900 students at Dallas 9 X 102 = 90 X 101 = 3 = 0.9 X 10 Measuring Scientific Notation Write 4500 in scientific notation with the following exponents: X 103 X 102 X 105 X 104 Measuring Scientific Notation Write 4500 in scientific notation with the following exponents: 4.5 X 103 45 X 102 0.045 X 105 0.45 X 104 Measuring Scientific Notation Examples: (2.0 x 102) + (3.0 x 103) = 3.2 X 103 (6.0 X 103) ÷ (3.0 x 10-5)=2.0X108 (2.0 x 107) - (6.3 x 105) = 1.9X107 Measuring Scientific Notation (4.0 x 105) x (3.0 x 10-1)= (6.0 x 108) ÷ (3.0 x 105)= (8.4x 1012) ÷ (8.4 x 109)= NOTE: 103 = 1 X 103 Measuring Scientific Notation (4.0 x 105) x (3.0 x 10-1)=1.2 X105 (6.0 x 108) ÷ (3.0 x 105)= 2 X 103 (8.4x 1012) ÷ (8.4 x 109)= 1 X 103 NOTE: 103 = 1 X 103 Measuring Accuracy and Precision • Accuracy – how close the average of a set of measurements is to the accepted value (AAA) • Precision – How close a set of measured values are to one another (reproducibility) • Always compare to a textbook value Measuring X X X X X X X X X X X XX X X X Measuring Percent Error Percent Error – Measure of accuracy % Error = Experimental – Accepted X 100 Accepted NOTE: “Experimental” =average of all trials Measuring A student measures the density of a sample of copper at 8.75 g/mL. The accepted value is 8.96 g/mL. Calculate the percent error. Measuring Error Analysis: Range Range - Measure of precision Range = highest trial – lowest trial Measuring Example 1 A student measures the density of a sample of lead and does four trials (11.3, 10.5, 11.9, 10.8 g/cm3). Calculate the range and comment on precision. Measuring Accuracy and Precision Students did trials to measure the density of a metal. The accepted density is 7.2 g/cm3. Were they accurate or precise? Set 1 Set 2 Set 3 7.21 7.25 7.18 6.40 7.90 7.30 6.45 6.52 6.48 Measuring Significant Figures 1. Def - All of the measured values plus one estimated place 2. Examples 6 cm 0.005 mm 1340 kg 6.0 cm 0.0050 mm 1340. kg 6.01 cm 0.00500 mm 1340.0 kg Measuring Numbers with a Decimal How many sig figs? Also, write in sci. notation: 3.44 cm 60.001 cm 430.0 cm 0.0032 cm 0.00320 cm Measuring Numbers without a Decimal 1. Often poor measurements 2. Examples: “Not left” 18,500 kg 120 ft Measuring Numbers without a Decimal How many sig figs? Also, write in scientific notation: 10,500 cm 240 cm 120,000 cm 4 cm 45 cm How many significant figures are in the following? Also, write the numbers in proper scientific notation. 1508 cm 20.003 lb 300 ft 300.0 ft 0.00705 m 0.007050 m 1250 1250. 1250.0 Measuring Significant Figures Round the following to three sig figs: 32.45 32.449 0.0067530 0.003904 11,980 Round to four significant figures: 598,937 0.00053254 5.37286 0.39201 0.39205 How many significant figures? 0.00200 7450 8.40 X 1010 0.0020 144.0 9.000 X 10-5 Round to three significant figures: 54.649999 300.847 200.49 0.00056732 0.0045282 1.456 X 10-4 8.605 X 107 100. 200 Measuring Significant Figures and Math 1. Math answers are only as good as the worst measurement. 2. Example: Determining the area of a room: 6.9 m by 10.478 m 3. Round AFTER you do the math. Measuring Significant Figures and Math Addition/Subtraction Rule - Keep the least number of decimal places. Examples: 7.56 0.0327 0.375 – 0.00068 + 14.2203 Measuring Significant Figures and Math Multiplication/Division Rule – Answer contains the least # of TOTAL significant figures Examples 23.4 X 32.25 = Measuring Significant Figures and Math 11.688 4.0 = 7 cm X 7 cm = 4.68 X 1016 9.1 X 10-5 = 1. Multiple Operations – Round when you change between add/sub and mult/div 2. Examples (0.56 X 11.73) + 22.34 = (6.5688) + 22.34 = (6.6) + 22.34 = 28.9 (12.45 – 11.643) X 2.68 = (0.807) X 2.68 = 0.81 X 2.68 = 2.1708 = 2.2 160 X 3.445 = 19.64 + 0.466 = 4.856 X 10102.0 X 102= (16.44 2.33) + 22.3 = (7.055793991) + 22.3 7.06 + 22.3 = 29.36 = 29.4 Measuring Significant Figures and Math 160 X 3.445 = 550 19.64 + 0.466 = 20.11 4.856 X 10102.0 X 102 = 2.4 X 108 (16.44 2.33) + 22.3 = 29.4 Measuring Warm-Up 19.64 - 14.465 = 320 X 0.04550 = 3.1415 X 1011 X 8.47 X 10-7= (12.7 X 10.43) + 23.8 = 0.00320 X 10-4 (write in proper sci. not.) Measuring Absolute Numbers Also called “exact” numbers Have an infinite number of significant figures Counting numbers and values in definitions. Examples: 23 students Diameter = 2r 1 km=1000m 5. NEVER use exact numbers for determining sf. 1. 2. 3. 4. Measuring Absolute numbers or measured values? Y= X3 1 m = 100 cm 2.85 grams 1 cm = 10 mm 37 apples 50 people 400 people Measuring Absolute Numbers If we divide 1.66 lbs of candy among 3 people, how much candy will each person get? (Ans: 0.553 lbs/person) What is the diameter of a circle whose radius is 3.835 m? (Ans: 7.670 m) Measuring 1. What is the diameter of a circle with a radius of 2.567 cm? 2. If we buy 1.84 pounds of coffee and divide it among three people, how much coffee will each person get? 3. How many centimeters is 7.565 meters? 4. How would you divide 12.35 kg of candy among eight children? Measuring Metric Qualitative – data with no number Quantitative – data with a number Measuring Metric 1. SI System – Le System International d’Unites 2. 1670 – Gabriel Mouton (French Vicar) 3. 1795 – Adopted by France Measuring Measuring Metric 4. Base ten scale 1000 m 100 m 10 m 1m 1m 1m 1m = = = = = = = 1 km (kilo) 1 hm (hecto) 1 dam (deca) 1m 10 dm (deci) 100 cm(centi) 1000 mm (milli) Measuring Measuring Metric Fundamental Units (MKS) Length meter Mass kilograms Time second Derived Units Volume liter (dm3) Energy Joules (kg m2/s2) Measuring Metric Factor Label method 55 cm = ? m 0.055 L = ? mL 0.00456 km = ? cm 550 cm2 = ? m2 25 miles/hr = ? m/s a. b. c. d. e. f. g. h. 129 hrs Days 0.468 mkm 825 cm2 in2 0.00230 L mL 0.468 m mm 1245 cm km 55.0 mi/hr km/hr 55.0 mi/hr m/min 129 hrs Days 0.468 mkm 825 cm2 in2 0.00230 L mL 0.468 m mm 1245 cm km 55 mi/hr km/hr 55 mi/hr m/min 5.38 days 0.000468 128 in2 2.30 mL 468 mm 0.01245 km 88.5 km/hr 1470 m/min Measuring Metric 1 km 1 hm 1 dam 1m 1 dm 1 cm 1 mm = = = = = = = 103 m 102 m 101 m 1m 10-1 10-2 10-3 m Convert using powers of ten 50 cm = ? m 5 mm = ? m 65 km = ? m 23.3 mL = ? L 0.0047 mm = ? m 0.876 L = ? mL 1. 2. 3. 4. 5. 6. 7. Round to 3 sf: 0.0050460 Calculate using sf (10.345 – 8.23) X 54 65.0 m/s =? miles/hr 584 cm3 = ? in3 234 cm = ? Feet 3.00 X 108 m/s = miles/s 45.0 L/s = gallons/min (1.00 inch = 2.54 cm) (1.609 km = 1.00 mile) (1.000 gallon = 3.785 L) 1. 2. 3. 4. 5. 6. 7. 0.00505 110 145 miles/hr 35.6 in3 7.68 Feet 3.00 X 108 m/s = 186 000 miles/s 713 gallons/min (1.00 inch = 2.54 cm) (1.609 km = 1.00 mile) (1.000 gallon = 3.785 L) Temperature Measuring Temperature Absolute Zero • All atomic and molecular motion stops • Coldest possible temperature? • Liquid Nitrogen = 77 K (-196 oC) • Dry Ice = 216 K (-56.6 oC) Measuring Measuring Measuring Planck Temperature = 1.417 x 1032 K (temperature of the Big Bang) Measuring Temperature Conversion Formulas F = 1.8 (oC) + 32 K = C + 273 C = K – 273 Measuring Temperature Ex: 24 oC 48oF 177 K = = = oF oC oC Measuring Temperature 102 oF -10.0 oC 25 oC 177 K 310 oF oC oF K oF K Measuring Temperature 102 oF -10.0 oC 25 oC 177 K 310 oF 39oC 14 oF 298 K -141 oF 427 K Measuring Temperature 25 oC 50 oF 310 K 10 K -15 oC oF K oC oC K Measuring Temperature 25 oC 50 oF 310 K 10 K -15 oC 77 oF 283 K(10 oC) 37 oC -263 oC 258 K Measuring Page 39 15 a) 0.77 b) 13.0 21 a) 5000 m d) 100 yd 23 a) 7 c) 32 b) 1400 ft2 b) 12.7 c) 1.49 d) 326 c) 1.21 in2 Measuring Page 40 (40-42, 53, 55, 57, 60) 42 a) 6.8 X 106 6800 6.8 b) 786 0.786 7.68X10-4 c) 4452 4.452 4.452 X 10-3 53) 384,300km 55) 0.376 qt 57) 114 g 60) 109 yd (10.9 yd) Measuring 23 a) b) c) 42 a) b) c) 7 12.7 1.49 6.8 X 106 786 4452 6800 0.786 4.452 6.8 7.68X10-4 4.452 X 10-3 Measuring Measuring Sig Figs Review WS 1 a=4b=3 c=2 d=4 e=3 2a) 20. e) 6.27 b) 960 f) 417 c) 55.2 g) 2.7 d) 5800 f=6 g=2 h=3 Measuring B2) 3ft=1yd, 10 dm = 1 m 1.00 gal = 3.78 L 2.20 lb = 1.00 kg B3) 15.5 miles B4) $2.16, 9.72 oz B5) 366 cm B6) $8.94 Measuring 29 4.23 X 105 4.338 X 102 2.0 X 10-3 8.8 X 102 8 X 10-5 8.2 X 107 7.5 X 1013 1.06 X 10-6 Measuring 39 1.58 X 10-10 2.29 X 1010 3.69 X 10-6 3.15 X 1012 3. Most precise = 26.202, most acc = 26.8 Measuring 5. a) 2 b) 3 c) 3 d) 3 e) 4 f) 5 g) 2 h) 2 9. a) 120 b) 28 c) 38,000 d) 0.47 e) 56 f) 0.040 g) 1,600,000 h) 320 11.a) 0.667 b) 0.400 c) 0.625 d) 3.25 15. a) 0.77 b) 13.0 c) 32 d) 326 24. a) 120 cm2 b) 394 ft2 c) 2 cm d) 2.3 in 25. a) 5000 m b) 1400 ft2 d) 1.21 in2 d) 100 yd 27. a) 7 b) 12.7 c) 1.49 28.a) 1.57 X102 b)1.57X10-1 c) 3.00 X10-2 Measuring d) 4.0 X107 e) 3.49 X10-2 f) 3.2 X 104 g) 3.2 X1010 h) 7.71 X10-4 i) 2.34 X 103 29. a) 4.23 X105 b) 4.338 X102 c) 2.0 X10-3 d) 8.8 X102 e) 8 X10-5 f) 8.20 X107 g) 7.5 X1013 h) 1.06 X10-6 32. a) 0.000475 b) 6550 c) 0.00788 d) 489,000 e) 4.75 f) 3.4 33.a) 0.064 b) 8340 c) 220 d) 0.00342 34. a) 4.89 X10-4 b) 4.56 X10-5 c) 7.8X 103 Measuring d)5.71 X10-2 e) 4.975 X108 f) 3.0 X 10-2 35. a) 7.8X10-10 b) 7.2X10-1 c) 3.450X1019 d) 2.8X1010 e) 6.9X10-14 f) 2.3X103 39. a) 1.58 X10-10 c) 3.69 X10-6 43.a) 4.56 X1016 c) 1.7 X10-14 b) 2.29 X1010 d) 3.15 X1012 b) 5 X10-9 d) 1.26 X1012 Write in Sci Notation Measuring 4 X 102 5 X 10-3 6 X 104 3.4 X 10-3 7.5 X 1012 6.457 X 10-2 5.6 X 10-5 4.5 X 102 Write the expanded number 0.000 05 2 000 000 000 0.144 150 000 000 000 0.000 000 244 300 000 0.00045 45 000 Calculate in Sci Not a) 3 X 105 b) 2 X 103 c) 4.3 X 103 d) 6 X 107 e) 2.5 X 10-6 f) 1.664 X10-3 g) 3.0 X 104 h) 8 X 10-4 i) 1.6 X 101 j) 1.16 X 107 How many signif figures? Calculate using SF a) 15.2 m) 91.0 b) 20. n) 4.1 c) 6 o) 0.0075 d) 19.4 e) 15 f) 3.1 g) 1.23 h) 4.27 i) 0.0102 j) 50 k) 49 l) 49.0 Multiple operations & SF a) 20. b) 960 c) 55.2 d) 5800 Abs. # Calculations a) 303 cm3 b) 756.3 cm c) 1.544 kg/child d) 0.65 m e) 5.134 cm f) 25.6 ml g) 553 cm2 h) 0.613 kg/person Metric Conversions Measuring a) 250 cm m) 5.678292 km b) c) d) e) f) g) h) i) j) k) l) 57 cm 0.42 m 420 mm 46.7 m 72,000 ml 2.3 cm 8.955 g 8.68 X 10-6 kg 0.654 g 6,000 mL 1.2 dm n) o) p) q) r) s) t) u) v) w) x) 0.088 L 19 mL 3.9 m 0.0234 L 45 mL 1.2 cm 0.072 g 0.0862 km 2470 cm 340 mL 4.8 cm y) 0.0012 mL z) 2.3 mL Temperature Conversions Measuring a) b) c) d) e) f) g) h) i) j) 298 K 25 oC 226 K -196 oC 62.2 oC 309 K 263 K 59 oC 127 oC 176 K k) l) m) n) o) p) q) 5273 K -271 oC 371 K 18.3 oC 239 K 33 oC 256 K 62. lbs g kg 1.72 780. 0.780 2.17 985 0.985 16.0 7260 7.26 71. Longer, 10.9 yards 102.a) 310 K b) 408 K c) -68oC d) -231 oF e) 311 K d) 248 K Complete the following chart (1.00 inch = 2.54 cm) Measuring inches cm m 4.75 824 0.537 How many Dekameters is 456 cm? Convert 60oF to Celsius and Kelvin Complete the following chart (1.00 inch = 2.54 cm) Measuring inches cm m 4.75 12.1 0.121 324 824 8.24 21.1 53.7 0.537 How many Dekameters is 456 cm? 0.456 dam Convert 60.0oF to Celsius and Kelvin 15.6oC, 289K A 43 Measuring G 400 (4 X 102) B 5.5 H 35 C 306.9 I 30 D 2.21 J 25 E 7.7 K 40 F 10.88 L 165