4.1 Gases
advertisement
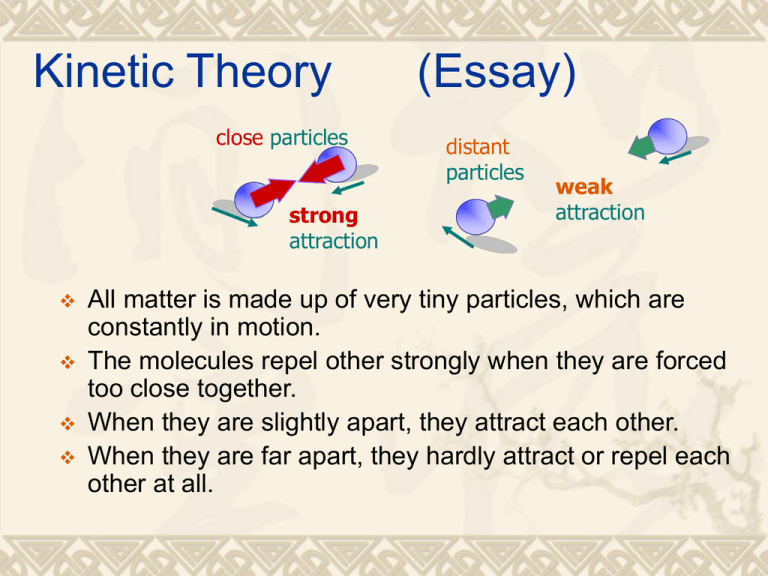
Kinetic Theory close particles strong attraction (Essay) distant particles weak attraction All matter is made up of very tiny particles, which are constantly in motion. The molecules repel other strongly when they are forced too close together. When they are slightly apart, they attract each other. When they are far apart, they hardly attract or repel each other at all. Interpreting temperature by kinetic theory The motion of the particles in a body depends on its temperature. When the temperature rises, particles vibrate more rapidly or move faster. Hence, the temperature of the body is a measure of the average kinetic energy of the particles. Two bodies have the same temperature if particles in each body have the same average kinetic energy. A gas model (Essay) Particles move around freely at high speed container Gases have no fixed volume and shape. They can quickly fill any space available. In a gas, particles are very far apart. Therefore, the attraction among them is weak. A gas model Particles move around freely at high speed container video The particles move at random at very high speeds (500 ms-1 at room temperature!). They collide with each other and bombard the walls of the container. When gas molecules hit the walls of container, they produce forces on the surface, which give rise to gas pressure. Gas pressure Pressure is defined as the perpendicular force exerted per unit area. Force perpendicular to the surface Pressure Area of surface F P A Pressure is measured in N m-2 or pascal (Pa). 1 Pa = 1 N m-2. Normal atmospheric pressure or 1 standard pressure = 1.01 x 105 Pa Example 1 Find the pressure exerted on the 5 kg block in each of the following cases. (a) 10 cm 5 cm Solution: P= F/A = 50 / (0.05 x 0.1) = 10000 Pa Example 1 Find the pressure exerted on the 5 kg block in each of the following cases. 10 cm (b) 5 cm 30o Solution: P= F/A (F = normal reaction) = 50 cos30o / (0.05 x 0.1) = 8660 Pa Example 2 The figure shows a cylinder with a piston. If the cross section area of the cylinder is 20 cm2, and find the pressure of the gas. Given that the atmospheric pressure is 1.01 x 105 Pa. Answer: 2.05 x 105 Pa 0.2 kg Measuring gas pressure A Bourdon gauge is used to measure gas pressures. Measuring gas pressure A bourdon gauge works in the same way as a paper tube whistle. When a paper tube whistle is blown, it uncoils. In a Bourdon gauge, the gas pressure makes the curved metal tube stretch out slightly. This causes the pointer to move round the scale. The higher the gas pressure, the more the pointer moves. Pressure, volume and temperature When a gas is heated, its pressure P, volume V and temperature T will change. We are going to investigate these relations. Boyle’s law (P-V relationship) In 1660, Robert Boyle investigated the relationship between the volume of a fixed amount of gas and its pressure, keeping the temperature of the gas constant. Video Experiment Press the air pump gently to change the pressure and the volume of the trapped gas in the glass tube without changing its temperature. Measure the pressure and the volume of the gas. Repeat the above step to get more sets of pressure and volume. Results P ∝ 1/V or PV = constant or P1V1 = P2V2 Boyle’s law: For a fixed mass of gas at a constant temperature, the product of its pressure and volume is constant. Example 2 The air pressure inside an inflated ball is 2 x 105 Pa. The ball is now squeezed to one-third of its original volume. Find the new pressure inside the ball. Solution: Let V be the original volume of the ball. By P1V1 = P2V2 2 x 105 V = P2(V/3) P2 = 6 x 105 Pa Thus, the new pressure is 6 x 105 Pa Charles’ law (V – T relationship) In this experiment, we keep the pressure of the gas constant and try to investigate the relation between its volume and temperature. Experiment Gas column Heat the water until its temperature is increased by 10oC. Record the volume and temperature of the trapped gas. Repeat the above step to get more sets of volume and temperature. The mercury thread should be able to move up and down the capillary tube freely. The friction between the mercury thread and the capillary tube should be small so that the pressure of the trapped gas is equal the atmospheric pressure. Charles’ law (V – T relationship) Gas column simulation Heat the water until its temperature is increased by 10oC. Record the volume and temperature of the trapped gas. Repeat the above step to get more sets of volume and temperature. The mercury thread should be able to move up and down the capillary tube freely. The friction between the mercury thread and the capillary tube should be small so that the pressure of the trapped gas is equal the atmospheric pressure. Absolute zero The straight line cuts the axis at 273oC which is known as the absolute zero of temperature. Absolute zero is the theoretical lowest attainable temperature. Theoretically, the gas at absolute zero occupies zero volume and exerts no pressure. However, in practice, it will liquefy before it can reach this temperature. Kelvin scale When the temperature is measured in Kelvin (K), the volume of the gas is directly proportional to its temperature shown in the figure below. volume V 0 Temperature T (K) Note: Absolute temperature (K) = Celsius temperature + 273 i.e. melting point of ice: 0oC = 273 K room temperature: 27oC = (27 + 273) K = 300 K boiling point of water: 100oC = (100 + 273) K = 373 K volume V Temperature T (K) 0 V T or V constant T V1 V2 or T1 T2 Charles’s law: For a fixed mass of gas at a constant pressure, its volume is directly proportional to its absolute temperature. Example 2 An inflated balloon contains 4 x 10-3 m3 of air at 27oC. It is put into a large tank of liquid nitrogen at -173 oC. What is the new volume of the balloon? Solution: By Charles’ law, V1/T1 = V2/T2 4 x 10-3 / (27 + 273) = V2 / (-173 + 273) V2 = 1.33 x 10-3 m3 Thus, the new volume of the balloon is 1.33 x 10-3 m3 Pressure law (P – T relationship) Experiment Heat the water unit its temperature increases by about 10oC. Stir the water thoroughly and wait for several minutes to allow the air in the flask to reach the temperature of the water. Measure the temperature of the air by the thermometer and its pressure by the Bourdon gauge. Repeat the above steps to get more sets of pressure and temperature. Results The pressure of the gas increases linearly with its temperature. When the temperature is measured in Kelvin (K), the pressure is directly proportional to its temperature shown in the figure below. pressure P 0 Temperature T (K) P1 P2 P constant or P T or T1 T2 T Pressure law: For a fixed mass of gas at a constant volume, its pressure is directly proportional to its absolute temperature. Example 3 When the flask used in the experiment is put into a bath of melting ice, the pressure is 8.7 x 104 Pa. What is the pressure when the flask is put into a bath of boiling water? Solution: Temperature of melting ice = 0 oC = 273 K Temperature of boiling water = 100 oC = 373 K By pressure law, P1/ T1 = P2 / T2 8.7 x 104 / 273 = P2 / 373 P2 = 1.19 x 105 Pa Thus, the new pressure is 1.19 x 105 Pa General gas law Boyle’s law: Charles’ law: Pressure law: All the gas laws can be summarized by a general equation: PV = constant if T is constant V/T = constant if P is constant P/T = constant if V is constant PV constant or T P1V1 P2V2 T1 T2 Example 4 A weather balloon contains 5 m3 of helium at the normal atmospheric pressure of 100 k Pa and at temperature of 27 C. What will be its volume when it rises to an altitude where the pressure is 80 k Pa and the temperature is 7 C? Solution: P1V1 P2V2 By T1 T2 (100 k)(5)/(27 + 273) = (80 k)(V2) / (7 + 273) V2 = 5.83 m3 Thus, its new volume is 5.83 m3 Equation of state and molar gas constant For 1 mole of gas (i.e. 6.02 x 1023 gas particles), PV R T (same for all gases) where R is called the universal molar gas constant. R = 8.31 J mole-1 K-1 For n moles of gas, PV nR PV nRT T Equation of state and molar gas constant If m is the mass of a gas and M is the mass of 1 mole of m gas, the number of mole n M m RT From PV nRT , we have PV M If N is the number of molecules in a gas and NA = Avogadro N number = 6.02 x 1023, the number n of mole NA N From PV nRT , we have PV RT NA . R = 8.31 J K-1 mol-1 Example 5 Show that the volume of 1 mole of gas occupied at s.t.p. is 22.4 litres. Note: s.t.p. stands for standard temperature and pressure. Standard temperature = 0oC or 273 K Standard pressure = 1 atmospheric pressure = 1.01 x 105 Pa Solution By PV = nRT (1.01 x 105)V = (1)(8.31)(273) V = 0.0224 m-3 or 22.4 litres Ideal gases An ideal gas is a gas that obeys Boyle’s law (PV is constant) for all pressures and temperatures. However, real gases such as hydrogen, oxygen, nitrogen or carbon dioxide are not ideal gases but they behave like an ideal gas at high temperature and low pressure. Example 6 Two insulated gas cylinders A and B are connected by a tube of negligible volume as shown. Each cylinder has an internal volume of 2 x 10-2 m3. Initially, the tap is closed and cylinder A contains 1.2 mol of an ideal gas at a temperature of 37oC. Cylinder B contains the same ideal gas at pressure 1.2 x 105 Pa and temperature 37 oC. (a) Calculate the amount, in mol, of the gas in cylinder B. Solution: Consider the gas in cylinder B By PV = nRT (1.2 x 105 )(2 x 10-2) = n(8.31)(37 + 273) n = 0.932 The amount of gas in cylinder B is 0.932 mol Example 6 Two insulated gas cylinders A and B are connected by a tube of negligible volume as shown. Each cylinder has an internal volume of 2 x 10-2 m3. Initially, the tap is closed and cylinder A contains 1.2 mol of an ideal gas at a temperature of 37oC. Cylinder B contains the same ideal gas at pressure 1.2 x 105 Pa and temperature 37 oC. (a) Calculate the pressure of the gas in cylinder A. Solution: Consider the gas in cylinder A By PV = nRT P(2 x 10-2) = (1.2)(8.31)(37 + 273) P = 1.55 x 105 Pa The pressure of gas in cylinder A is 1.55 x 105 Pa Example 6 Two insulated gas cylinders A and B are connected by a tube of negligible volume as shown. Each cylinder has an internal volume of 2 x 10-2 m3. Initially, the tap is closed and cylinder A contains 1.2 mol of an ideal gas at a temperature of 37oC. Cylinder B contains the same ideal gas at pressure 1.2 x 105 Pa and temperature 37 oC. (b) determine the final pressure of the gas in the cylinder. Solution By PV = nRT P(4 x 10-2) = (1.2 + 0.962)(8.31)(37 + 273) P = 1.37 x 105 Pa 1.2 mol 0.962 mol Example 7 There are two containers X and Y filled with the same type of ideal gas as shown. They are connected by a tube. A steady state is obtained with X held at 100 K and Y at 400 K. If the volume of X is half that of Y and the mass of gas in X is m, what is the mass of gas in Y, in terms of m? Solution: Let P be the pressure of the gas and V be the volume of container X For the gas in container Y, P(2V) = nYR(400) --- (1) For the gas in container X, P(V) = nXR(100) --- (2) (1)/(2): 2 = 4nY/nX nY = ½ nX Mass of gas in Y = ½ mass of gas in X = ½ m Example 7 root mean square Find (a) the mean (b) mean square, and (c) the root mean square of 1, 3, 5, 7 and 9. Solution: Mean = (1 + 3 + 5 + 7 + 9) / 5 = 5 Mean square = (12 + 32 + 52 + 72 + 92)/5 = (1 + 9 + 25 + 49 + 81) / 5 = 33 Root mean square = [(12 + 32 + 52 + 72 + 92)/5] ½ = 5.74 Example 8 It is given that the density of hydrogen gas at s.t.p. is 0.09 kg m-3. Find the r.m.s. speed of hydrogen molecules. Solution: By P = ⅓(rcr.m.s.2) 1.01 x 105 = ⅓(0.09cr.m.s.2) cr.m.s. = 1835 ms-1 Distribution of molecular speeds Particles move around container freely at high speed Simulation 1 A gas contains a large number of molecules in rapid motions. In each collision between molecules, some molecules gain energy while others lose energy. As a result, each molecule has a different speed. Simulation 2 Maxwell distribution Note: Maxwell distribution is not symmetric. When temperature increases, the distribution curve flattens out but the area under the curve remains unchanged. Most probable speed c0: speed possessed by the most number of molecules Number of molecules with speed c Mean speed cm: mean speed of molecules Maxwell distribution c c c cm 1 2 N N Root mean square speed cr.m.s.: 0 co cm cr.m.s. It is found that c0 < cm < cr.m.s. c/m s-1 c1 c 2 c N N 2 c r .m. s . 2 2 Temperature, molar mass and root mean square speed 1 PV Nmc r2.m.s and 3 1 2 Nmc we have r .m . s nRT 3 From PV nRT For 1 mole of gas, n = 1, N = NA and the mass of the gas = molar mass Mm = mNA 1 Nmc r2.m.s nRT 3 3RT 1 2 N A mc r .m.s RT ⇒ c r .m.s ⇒ Mm 3 By cr .m.s 3RT Mm Note: cr.m.s T cr.m.s. increases with temperature. cr.m.s. decreases with the mass of the molecule or molar mass of the gas. 1 c r . m. s Mm Example 8 Find the root mean square speed of hydrogen molecules at 27oC. It is given that R = 8.31 J K-1 mol-1. Solution: By cr .m.s 3RT Mm cr.m.s. = (3 x 8.31 x 300 / 0.002)½ = 1934 ms-1 Example 9 Find the ratio of the root mean square speed of hydrogen to that of oxygen. 3RT Mm By c r .m.s For hydrogen: ch = (3RT/0.002) ½ For oxygen: co = (3RT/0.032) ½ ch / co = (0.032/0.002)½ = 4 the ratio of the root mean square speed of hydrogen to that of oxygen is 4. Translational K.E. and Rotational K.E. Translational motion Rotational motion Translational + rotational motion Monoatomic and diatomic gases Monoatomic means having only one atom in the molecule. All of the noble gases are monoatomic. Diatomic means having two atoms in the molecule. Examples are oxygen, nitrogen kinetic energy of a monoatomic gas Translational K.E. = ½ mc2 Rotational motion = 0 (neglected) Translational K.E. of a monoatomic gas 1 From PV Nm c 2 and PV nRT 3 1 Nmc 2 nRT we have 3 For 1 mole of gas, n = 1, N = NA and the mass of the gas = molar mass Mm = mNA 1 2 1 2 N m c RT Nmc nRT ⇒ A 3 3 Average translational kinetic energy of a molecule R 1 2 3 R 3 23 1 k 1 . 38 10 JK where T kT = mc NA 2 2 NA 2 which is known as Boltzmann constant. Note: All types of gas molecule have the same average translational kinetic energy at the same temperature. ( 1 mc 2 3 kT ) 2 2 For diatomic gases such as hydrogen, oxygen and nitrogen, the average kinetic energy 5 (translational + rotational) of a molecule = kT . 2 (Out of syllabus) Avogadro’s law Avogadro’s law states that under the same temperature and pressure, equal volumes of different gases contain an equal number of molecules. Proof Consider 2 gases under the same temperature, pressure and volume. Let N1 and N2 be the number of molecules in gas 1 and gas 2 respectively. 1 For gas 1: P1V1 N 1 m1 c1 2 3 1 2 For gas 2: P2V2 N 2 m2 c 2 3 Same volume and pressure ⇒ V1 = V2 and P1 = P2 1 1 2 2 N m c N m c ∴ 1 1 1 2 2 2 3 3 Same temperature ⇒ same average kinetic energy of a molecule ∴ 1 2 mc m c 2 1 1 1 2 2 2 2 1 2 1 2 N m c N 1 m1 c1 2 2 2 ⇒ N1 = N2 3 3 Dalton’s law of partial pressures Dalton's law of partial pressures states that the total pressure exerted by a mixture of gases is the sum of the pressures exerted by each gas if it were allowed to occupy the whole volume alone. p1 p p2 p = p1 + p2 Dalton’s law of partial pressures PV = nRT p1 p2 n1 RT P1 V n2 RT P2 V P = P1 + P2 p P n1 n2 RT V