H 2 O + (aq)
advertisement

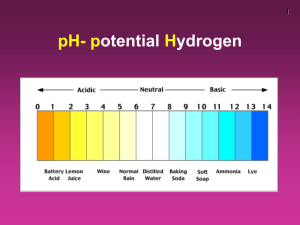
Chapter 18 Acid-Base Equilibria Acid-Base Equilibria 18.1 Acids and Bases in Water 18.2 Autoionization of Water and the pH Scale 18.3 Proton Transfer and the Brønsted-Lowry Acid-Base Definition 18.4 Solving Problems Involving Weak-Acid Equilibria 18.5 Weak Bases and Their Relations to Weak Acids 18.6 Molecular Properties and Acid Strength 18.7 Acid-Base Properties of Salt Solutions 18.8 Generalizing the Brønsted-Lowry Concept: The Leveling Effect 18.9 Electron-Pair Donation and the Lewis Acid-Base Definition The Nature of Acids and Bases: Acids: Acids taste sour. React with metals and produce H gas. turns blue litmus red pH < 7 * Bases: Bases taste bitter. They are slippery. turns red litmus blue. pH >7 Arrhenius concept Acids produce H+ ions. Bases produce OH- ions. This concept is limited because it applies to only aqueous solution and defines only OH containing bases. Neutralization: acid + base _______salt + water. Acid Dissociation Constant (Ka) Write Ka expression for strong and weak acids. Figure 18.1 The extent of dissociation for strong acids. Strong acid: HA(g or l) + H2O(l) H2O+(aq) + A-(aq) Figure 18.2 The extent of dissociation for weak acids. Weak acid: HA(aq) + H2O(l) H2O+(aq) + A-(aq) Figure 18.3 1M HCl(aq) Reaction of zinc with a strong and a weak acid. 1M CH3COOH(aq) Strong acids dissociate completely into ions in water. HA(g or l) + H2O(l) H3O+(aq) + A-(aq) Kc >> 1 Weak acids dissociate very slightly into ions in water. HA(aq) + H2O(l) H3O+(aq) + A-(aq) Kc << 1 The Acid-Dissociation Constant [H3O+][A-] Kc = [H2O][HA] Kc[H2O] = Ka = stronger acid higher [H3O+] larger Ka [H3O+][A-] [HA] smaller Ka lower [H3O+] weaker acid Classifying the Relative Strengths of acids and Bases: Strong acids: – HCl, HBr, HI – Oxo acids. HNO3, H2SO4, HClO4 Weak acids: – HF – HCN , H2S (H not bonded to O or halogen) – Oxo acids. HClO, HNO2, H3PO4 Carboxylic acids. CH3COOH Classifying the Relative Strengths of acids and Bases: Strong bases: – M2O, MOH M= (group 1A metal) – MO , M(OH)2 M=group 2A metal Weak bases: (N atom and lone pair of electrons) – NH3 – Amines. ACID STRENGTH SAMPLE PROBLEM 18.1: PROBLEM: Classify each of the following compounds as a strong acid, weak acid, strong base, or weak base. (a) H2SeO4 PLAN: Classifying Acid and Base Strength from the Chemical Formula (b) (CH3)2CHCOOH (c) KOH (d) (CH3)2CHNH2 Pay attention to the text definitions of acids and bases. Look at O for acids as well as the -COOH group; watch for amine groups and cations in bases. SOLUTION: (a) Strong acid - H2SeO4 - the number of O atoms exceeds the number of ionizable protons by 2. (b) Weak acid - (CH3)2CHCOOH is an organic acid having a -COOH group. (c) Strong base - KOH is a Group 1A(1) hydroxide. (d) Weak base - (CH3)2CHNH2 has a lone pair of electrons on the N and is an amine. Autoionization of water and the pH scale Water dissociates into its ions. Autoionization of Water and the pH Scale + H2O(l) H2O(l) + H3O +(a q) OH-(aq) H2O(l) + H2O(l) Kc = H3O+(aq) + OH-(aq) [H3O+][OH-] [H2O]2 The Ion-Product Constant for Water Kc[H2O]2 = Kw = [H3O+][OH-] = 1.0 x 10-14 at 250C A change in [H3O+] causes an inverse change in [OH-]. In an acidic solution, [H3O+] > [OH-] In a basic solution, [H3O+] < [OH-] In a neutral solution, [H3O+] = [OH-] Figure 18.4 The relationship between [H3O+] and [OH-] and the relative acidity of solutions. [H3O+] Divide into Kw [OH-] [H3O+] > [OH-] [H3O+] = [OH-] [H3O+] < [OH-] ACIDIC SOLUTION NEUTRAL SOLUTION BASIC SOLUTION SAMPLE PROBLEM 18.2: PROBLEM: PLAN: Calculating [H3O+] and [OH-] in an Aqueous Solution A research chemist adds a measured amount of HCl gas to pure water at 250C and obtains a solution with [H3O+] = 3.0x10-4M. Calculate [OH-]. Is the solution neutral, acidic, or basic? Use the Kw at 250C and the [H3O+] to find the corresponding [OH-]. SOLUTION: K = 1.0x10-14 = [H O+] [OH-] so w 3 [OH-] = Kw/ [H3O+] = 1.0x10-14/3.0x10-4 = 3.3x10-11M [H3O+] is > [OH-] and the solution is acidic. pH scale pH log[H+] pH in water ranges from 0 to 14. Kw = 1.00 1014 = [H+] [OH] pKw = 14.00 = pH + pOH As pH rises, pOH falls (sum = 14.00). pH=7-neutral, pH>7-basic, pH<7acidic pH scale Acidic solns have a higher pOH. pK=-log K equation reaches equ, mostly products present, low pK (high K) Reverse of the above statement is also true. pH is measure using pH meter, pH paper or acid-base indicator. Figure 18.7 Methods for measuring the pH of an aqueous solution pH (indicator) paper pH meter Figure 18.5 The pH values of some familiar aqueous solutions pH = -log [H3O+] Table 18.3 The Relationship Between Ka and pKa Acid Name (Formula) Ka at 250C pKa 1.02x10-2 1.991 Nitrous acid (HNO2) 7.1x10-4 3.15 Acetic acid (CH3COOH) 1.8x10-5 4.74 Hypobromous acid (HBrO) 2.3x10-9 8.64 1.0x10-10 10.00 Hydrogen sulfate ion (HSO4-) Phenol (C6H5OH) Figure 18.6 The relations among [H3O+], pH, [OH-], and pOH. Problems P.3.Calculate pH and pOH at 25 C for: 1.0 M H+ P.4.pH=6.88, Calculate [ H+] and [ OH-] for this sample. P.5.Calculate pH of 0.10 M HNO3 P.6.Calculate pH of 1.0 x 10-10 HCl. Bronsted-Lowry model: An acid is a proton (H+ ) donor. A base is a proton acceptor. * A conjugate acid-base pair only differs by one H. HCl + H2O _________ H3O+ ClAcid base conj acid conj base HA(aq) + H2O (l) H3O+ (aq) +A-(aq) CA1 CB2 CA2 CB1 Bronsted-Lowry model: conjugate base: everything that remains of the acid molecule after a proton is lost. Has one H less and one more minus charge than the acid. conjugate acid: formed when the proton is transferred to the base. Has one more H and one less charge than the base. Do Follow up problem-18.4-Page.779 Brønsted-Lowry Acid-Base Definition An acid is a proton donor, any species which donates a H+. A base is a proton acceptor, any species which accepts a H+. An acid-base reaction can now be viewed from the standpoint of the reactants AND the products. An acid reactant will produce a base product and the two will constitute an acid-base conjugate pair. Table 18.4 The Conjugate Pairs in Some Acid-Base Reactions Conjugate Pair Acid + Base Base + Acid Conjugate Pair Reaction 1 HF + H2O F- + H3O+ Reaction 2 HCOOH + CN- HCOO- + HCN Reaction 3 NH4+ + CO32- NH3 + HCO3- Reaction 4 H2PO4- + OH- HPO42- + H2O Reaction 5 H2SO4 + N2H5+ HSO4- + N2H62+ Reaction 6 HPO42- + SO32- PO43- + HSO3- Proton transfer as the essential feature of a BrønstedLowry acid-base reaction. Figure 18.8 Lone pair binds H+ + + HCl H 2O (acid, H+ donor) Cl- H 3 O+ (base, H+ acceptor) Lone pair binds H+ + + NH3 (base, H+ acceptor) H2O (acid, H+ donor) NH4+ OH- SAMPLE PROBLEM 18.4: PROBLEM: Identifying Conjugate Acid-Base Pairs The following reactions are important environmental processes. Identify the conjugate acid-base pairs. (a) H2PO4-(aq) + CO32-(aq) (b) H2O(l) + SO32-(aq) PLAN: HPO42-(aq) + HCO3-(aq) OH-(aq) + HSO3-(aq) Identify proton donors (acids) and proton acceptors (bases). conjugate pair2 conjugate pair 1 SOLUTION: (a) H2PO4-(aq) + CO32-(aq) proton donor proton acceptor HPO42-(aq) + HCO3-(aq) proton acceptor conjugate pair2 conjugate pair1 (b) H2O(l) + SO32-(aq) proton proton donor acceptor proton donor OH-(aq) + HSO3-(aq) proton acceptor proton donor SAMPLE PROBLEM 18.5: PROBLEM: Predicting the Net Direction of an Acid-Base Reaction Predict the net direction and whether Ka is greater or less than 1 for each of the following reactions (assume equal initial concentrations of all species): (a) H2PO4-(aq) + NH3(aq) (b) H2O(l) + HS-(aq) PLAN: HPO42-(aq) + NH4+(aq) OH-(aq) + H2S(aq) Identify the conjugate acid-base pairs and then consult Figure 18.10 (button) to determine the relative strength of each. The stronger the species, the more preponderant its conjugate. SOLUTION: (a) H2PO4-(aq) + NH3(aq) stronger acid stronger base HPO42-(aq) + NH4+(aq) weaker base weaker acid Net direction is to the right with Kc > 1. (b) H2O(l) + HS-(aq) OH-(aq) + H2S(aq) weaker acid weaker base stronger base stronger acid Net direction is to the left with Kc < 1. Figure 18.9 Strengths of conjugate acidbase pairs Solving Problems Involving Weak-Acid Equilibria. Write balanced equation and Ka expression. Make an ICE table. Make required assumptions. Substitute values and solve for x. Verify assumptions by calculating % error. Problems P.7. Calculate the pH of 1.00 M aqueous solution of HF. Ka=7.2 x 104 P.8 Calculate the pH of 0.100 M aq solution of HOCl. Ka=3.5 x 10-8 SAMPLE PROBLEM 18.6: PROBLEM: PLAN: Finding the Ka of a Weak Acid from the pH of Its Solution Phenylacetic acid (C6H5CH2COOH, simplified here as HPAc) builds up in the blood of persons with phenylketonuria, an inherited disorder that, if untreated, causes mental retardation and death. A study of the acid shows that the pH of 0.12M HPAc is 2.62. What is the Ka of phenylacetic acid? Write out the dissociation equation. Use pH and solution concentration to find the Ka. Assumptions: SOLUTION: With a pH of 2.62, the [H3O+]HPAc >> [H3O+]water. [PAc-] ≈ [H3O+]; since HPAc is weak, [HPAc]initial ≈ [HPAc]initial [HPAc]dissociation HPAc(aq) + H2O(l) Ka = [H3O+][PAc-] [HPAc] H3O+(aq) + PAc-(aq) SAMPLE PROBLEM 18.6: Finding the Ka of a Weak Acid from the pH of Its Solution continued Concentration(M) Initial Change Equilibrium H2O(l) H3O+(aq) + 0.12 - 1x10-7 0 -x - +x +x 0.12-x - x +(<1x10-7) x HPAc(aq) + PAc-(aq) [H3O+] = 10-pH = 2.4x10-3 M which is >> 10-7 (the [H3O+] from water) x ≈ 2.4x10-3 M ≈ [H3O+] ≈ [PAc-] So Ka = (2.4x10-3) (2.4x10-3) [HPAc]equilibrium = 0.12-x ≈ 0.12 M = 4.8 x 10-5 0.12 Be sure to check for % error. [H3 O+] from water; [HPAc]dissn; 1x10-7M x100 = 4x10-3 % 2.4x10-3M 2.4x10-3M x100 = 2.0 % 0.12M SAMPLE PROBLEM 18.7: PROBLEM: PLAN: Determining Concentrations from Ka and Initial [HA] Propanoic acid (CH3CH2COOH, which we simplify and HPr) is an organic acid whose salts are used to retard mold growth in foods. What is the [H3O+] of 0.10M HPr (Ka = 1.3x10-5)? Write out the dissociation equation and expression; make whatever assumptions about concentration which are necessary; substitute. Assumptions: For HPr(aq) + H2O(l) H3O+(aq) + Pr-(aq) x = [HPr]diss = [H3O+]from HPr= [Pr-] Ka = [H3O+][Pr-] [HPr] SOLUTION: Concentration(M) Initial Change Equilibrium HPr(aq) + H2O(l) H3O+(aq) + Pr-(aq) 0.10 - 0 0 -x - +x +x 0.10-x - x x Since Ka is small, we will assume that x << 0.10 SAMPLE PROBLEM 18.7: Determining Concentrations from Ka and Initial [HA] continued 1.3x10-5 = [H3O+][Pr-] [HPr] x (0.10)(1.3x105 ) (x)(x) = 0.10 = 1.1x10-3 M = [H3O+] Check: [HPr]diss = 1.1x10-3M/0.10 M x 100 = 1.1% Percent dissociation: For a weak acid % dissociation increases as the acid becomes more dilute. For solution of any weak acid, [ H+] decreases as [HA]0 decreases, but % dissociation increases as [HA]0 increases. Problems P.11.Calculate the % dissociation of acetic acid (Ka= 1.8 x 10-5 ) 1.00 M and 0.100 M solutions. P.12.In a 0.100 M HC3H5O3 aqueous solution, lactic acid is 3.7 % dissociated. Calculate ka for the acid. [HA]dissociated Percent HA dissociation = x 100 [HA]initial Polyprotic acids acids with more than more ionizable proton H3PO4(aq) + H2O(l) H2PO4 -(aq) + H3 O+(aq) Ka1 = [H3O+][H2PO4-] [H3PO4] = 7.2x10-3 H2PO4 -(aq) + H2O(l) HPO4 2-(aq) + H3 O+(aq) Ka2 = [H3O+][HPO42-] [H2PO4-] = 6.3x10-8 HPO4 2-(aq) + H2O(l) PO4 3-(aq) + H3 O+(aq) Ka1 > Ka2 > Ka3 Ka3 = [H3O+][PO43-] [HPO42-] = 4.2x10-13 ACID STRENGTH Polyprotic Acids They can furnish more than one proton (H+) to the solution. Characteristics: 1. Ka values are much smaller than the first value , so only the first dissociation step makes a significant contribution to equilibrium concentration of H+ . 2. For sulfuric acid, it behaves as a strong acid in the first dissociation step and a weak acid in the second step, where its contribution of H+ ions can be neglected. Use quadratic equation to solve such kind of a problem. Problems P.13.Calculate the pH of a 5.0 M H3PO4 solution and the equilibrium concentration of the species H3PO4 , H2PO4-, HPO42-, PO43P.14.Calculate the pH of 1.0 M sulfuric acid solution. SAMPLE PROBLEM 18.8: PROBLEM: PLAN: Calculating Equilibrium Concentrations for a Polyprotic Acid Ascorbic acid (H2C6H6O6; H2Asc for this problem), known as vitamin C, is a diprotic acid (Ka1 = 1.0x10-5 and Ka2 = 5x10-12) found in citrus fruit. Calculate [H2Asc], [HAsc-], [Asc2-], and the pH of 0.050M H2Asc. Write out expressions for both dissociations and make assumptions. Ka1 >> Ka2 so the first dissociation produces virtually all of the H3O+. Ka1 is small so [H2Asc]initial ≈ [H2Asc]diss After finding the concentrations of various species for the first dissociation, we can use them as initial concentrations for the second dissociation. SOLUTION: H2Asc(aq) + H2O(l) HAsc-(aq) + H2O(l) HAsc-(aq) + H3O+(aq) Asc2-(aq) + H3 O+(aq) Ka1 = Ka2 = [HAsc-][H3O+] [H2Asc] [Asc2-][H3O+] [HAsc-] = 1.0x10-5 = 5x10-12 SAMPLE PROBLEM 18.8: Calculating Equilibrium Concentrations for a Polyprotic Acid continued Concentration(M) Initial H2Asc(aq) + H2O(l) 0.050 - 0 0 -x - +x +x 0.050 - x - x x Change Equilibrium HAsc-(aq) + H3O+(aq) Ka1 = [HAsc-][H3O+]/[H2Asc] = 1.0x10-5 = (x)(x)/0.050 M 5 x (0.050)(1.0x10 ) Concentration(M) Initial HAsc-(aq) + H2O(l) 7.1x10-4M Change Equilibrium x x = 7.1x10-4 M -x 7.1x10-4 - x (7.4x105 )(5x1012 ) pH = -log(7.1x10-4) = 3.15 Asc2-(aq) + H3O+(aq) - 0 - +x +x - x x = 6x10-8 M 0 Bases “Strong” and “weak” are used in the same sense for bases as for acids. strong = complete dissociation (hydroxide ion supplied to solution) NaOH(s) Na+(aq) + OH(aq) weak = very little dissociation (or reaction with water) H3CNH2(aq) + H2O(l) H3CNH3+(aq) + OH(aq) [B] BASE STRENGTH Kb = [BH+][OH-] Problems P.15.Calculate pH of 5.0 x 10-2 M NaOH solution. P.16.Calculate the pH for a 1.50 M solution of ammonia (Kb=1.8 x 10-5 ) Figure 18.11 Abstraction of a proton from water by methylamine. Lone pair binds H+ + CH3NH2 H 2O Methylamine Base in water + CH3NH3+ methylammonium ion OH- SAMPLE PROBLEM 18.9: PROBLEM: PLAN: Determining pH from Kb and Initial [B] Dimethylamine, (CH3)2NH, a key intermediate in detergent manufacture, has a Kb of 5.9x10-4. What is the pH of 1.5M (CH3)2NH? Perform this calculation as you did those for acids. Keep in mind that you are working with Kb and a base. (CH3)2NH(aq) + H2O(l) (CH3)2NH2+(aq) + OH-(aq) Assumptions: Kb >> Kw so [OH-]from water is neglible [(CH3)2NH2+] = [OH-] = x ; [(CH3)2NH2+] - x ≈ [(CH3)2NH]initial SOLUTION: Concentration Initial Change Equilibrium (CH3)2NH(aq) + H2O(l) (CH3)2NH2+(aq) + OH-(aq) 1.50M - 0 0 -x - +x +x 1.50 - x - x x SAMPLE PROBLEM 18.9: Determining pH from Kb and Initial [B] continued Kb = 5.9x10-4 = [(CH3)2NH2+][OH-] (x) (x) 5.9x10-4 = [(CH3)2NH] x = 3.0x10-2M = [OH-] 1.5M Check assumption: 3.0x10-2M/1.5M x 100 = 2% [H3O+] = Kw/[OH-] = 1.0x10-14/3.0x10-2 = 3.3x10-13M pH = -log 3.3x10-13 = 12.48 SAMPLE PROBLEM 18.10: Determining the pH of a Solution of APROBLEM: PLAN: Sodium acetate (CH3COONa, or NaAc for this problem) has applications in photographic development and textile dyeing. What is the pH of 0.25M NaAc? Ka of acetic acid (HAc) is 1.8x10-5. Sodium salts are soluble in water so [Ac-] = 0.25M. Write the association equation for acetic acid; use the Ka to find the Kb. SOLUTION: Concentration Ac-(aq) + H2O(l) Initial 0.25M - 0 0 -x - +x +x 0.25M-x - x x Change Equilibrium Kb = [HAc][OH-] [Ac-] HAc(aq) + OH-(aq) = Kw Ka Kb = 1.0x10-14 1.8x10-5 = 5.6x10-10M SAMPLE PROBLEM 18.10: Determining the pH of a Solution of Acontinued [Ac-] = 0.25M-x ≈ 0.25M Kb = [HAc][OH-] [Ac-] 5.6x10-10 = x2/0.25M x = 1.2x10-5M = [OH-] Check assumption: 1.2x10-5M/0.25M x 100 = 4.8x10-3 % [H3O+] = Kw/[OH-] = 1.0x10-14/1.2x10-5 = 8.3x10-10M pH = -log 8.3x10-10M = 9.08 Molecular Properties and Acid strength: Trends in acid strength of Nonmetal Hydrides: Across a period nonmetal hydride acid strength increases. Down a group nonmetal hydride acid strength increases. Bond strength decreases, acidity increases Figure 18.12 The effect of atomic and molecular properties on nonmetal hydride acidity. 6A(16) 7A(17) H 2O HF H 2S HCl H2Se HBr H2Te HI Electronegativity increases, acidity increases Trends in Acid strength of Oxoacids 1. For oxoacids with same number of O around E , acid strength increases with electronegativity of E. 2. For oxoacids with different number of O around E, acid strength increases with number of O atoms. Figure 18.13 H O The relative strengths of oxoacids. I > H O Br > H O O H O Cl << H O Cl O O Cl Table 18.7 Ka Values of Some Hydrated Metal Ions at 250C Hydrated Ion Ka Fe3+ Fe(H2O)63+(aq) 6 x 10-3 Sn2+ Sn(H2O)62+(aq) 4 x 10-4 Cr3+ Cr(H2O)63+(aq) 1 x 10-4 Al3+ Al(H2O)63+(aq) 1 x 10-5 Cu2+ Cu(H2O)62+(aq) 3 x 10-8 Pb2+ Pb(H2O)62+(aq) 3 x 10-8 Zn2+ Zn(H2O)62+(aq) 1 x 10-9 Co2+ Co(H2O)62+(aq) 2 x 10-10 Ni2+ Ni(H2O)62+(aq) 1 x 10-10 ACID STRENGTH Free Ion Figure 18.13 The acidic behavior of the hydrated Al3+ ion. Electron density drawn toward Al3+ Nearby H2O acts as base H 3 O+ H 2O Al(H2O)63+ Al(H2O)5OH2+ Acid-Base properties of Salt: Salt is an ionic compound. Ka xKb=Kw SAMPLE PROBLEM 18.11: Predicting Relative Acidity of Salt Solutions PROBLEM: Predict whether aqueous solutions of the following are acidic, basic, or neutral, and write an equation for the reaction of any ion with water: (a) Potassium perchlorate, KClO4 (b) Sodium benzoate, C6H5COONa (c) Chromium trichloride, CrCl3 PLAN: (d) Sodium hydrogen sulfate, NaHSO4 Consider the acid-base nature of the anions and cations. Strong acid-strong base combinations produce a neutral solution; strong acid-weak base, acidic; weak acid-strong base, basic. SOLUTION: (a) The ions are K+ and ClO4- , both of which come from a strong base(KOH) and a strong acid(HClO4). Therefore the solution will be neutral. (b) Na+ comes from the strong base NaOH while C6H5COO- is the anion of a weak organic acid. The salt solution will be basic. (c) Cr3+ is a small cation with a large + charge, so it’s hydrated form will react with water to produce H3O+. Cl- comes from the strong acid HCl. Acidic solution. (d) Na+ comes from a strong base. HSO4- can react with water to form H3O+. So the salt solution will be acidic. SAMPLE PROBLEM 18.12: Predicting the Relative Acidity of Salt Solutions from Ka and Kb of the Ions PROBLEM: PLAN: Determine whether an aqueous solution of zinc formate, Zn(HCOO)2, is acidic, basic, or neutral. Both Zn2+ and HCOO- come from weak conjugates. In order to find the relatively acidity, write out the dissociation reactions and use the information in Tables 18.2 and 18.7. SOLUTION: Zn(H2O)62+(aq) + H2O(l) HCOO-(aq) + H2O(l) Zn(H2O)5OH+(aq) + H3O+(aq) HCOOH(aq) + OH-(aq) Ka Zn(H2O)62+ = 1x10-9 Ka HCOO- = 1.8x10-4 ; Kb = Kw/Ka = 1.0x10-14/1.8x10-4 = 5.6x10-11 Ka for Zn(H2O)62+ >>> Kb HCOO-, therefore the solution is acidic. Molecules as Lewis Acids An acid is an electron-pair acceptor. A base is an electron-pair donor. F B F F acid F H + H N HH base B F F N HH adduct M(H2O)42+(aq) M2+ H2O(l) adduct Figure 18.15 The Mg2+ ion as a Lewis acid in the chlorophyll molecule. SAMPLE PROBLEM 18.13: Identifying Lewis Acids and Bases PROBLEM: PLAN: Identify the Lewis acids and Lewis bases in the following reactions: (a) H+ + OH- H2O (b) Cl- + BCl3 BCl4- (c) K+ + 6H2O K(H2O)6+ Look for electron pair acceptors (acids) and donors (bases). SOLUTION: acceptor (a) H+ + OHdonor H2O donor (b) Cl- + BCl3 BCl4- acceptor acceptor (c) K+ + 6H2O K(H2O)6+ donor

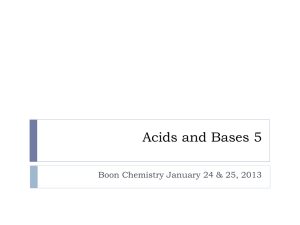

![pH = - log [H + ]](http://s2.studylib.net/store/data/005622524_1-002df1ea50d2a849b15deb604928664e-300x300.png)