4 Units of Measurement_ Precision vs Accuracy
advertisement

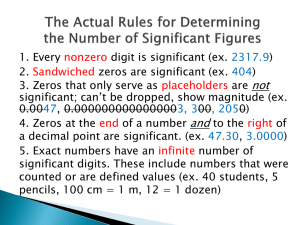
Units of Measurement Precision vs Accuracy Sections 1.3 and 1.4 Types of Observations and Measurements We make QUALITATIVE observations of reactions — changes in color and physical state. We also make QUANTITATIVE observations that involve MEASUREMENTS with numbers and units. NO NAKED NUMBERS A measurement always has two parts: A value (this is the number) A unit of measure (this tells what you have) Example: 200 meters; 15 ml; 13.98 grams In Chemistry we use SI Units of measure International System of Units These are the base units that we will be working with: From these base units, we can combine them using mathematical operations to obtain derived units. Some of the derived units we will be using include: Density (g/ml) Area (m2) Volume (m3) Prefixes Used in the SI System . Metric Prefixes Kilo- means 1000 of that unit 1 kilometer (km) = 1000 meters (m) Centi- means 1/100 of that unit 1 meter (m) = 100 centimeters (cm) 1 dollar = 100 cents Milli- means 1/1000 of that unit 1 meter (m) = 1000 millimeters (mm) To convert from one metric unit to another – remember this pneumonic: King Henry Died (Unexpectedly) Drinking Chocolate Milk You must also know… …how to convert within the Metric System. Here’s a good device: On your paper draw a line and add 7 tick marks: Next: Above the tick marks write the abbreviations for the King Henry pneumonic: k h d (u ) d m l g Write the units in the middle under the “U”. c m Let’s add the labels for meter, liters, and grams: k h d u d c m km hm dam m dm cm mm kl hl dal l dl cl ml kg hg dag g dg cg mg Deca can also be dk or da How to use this device: 1. Look at the problem. Look at the unit that has a number. On the device put your pencil on that unit. 2. 3. Move to new unit, counting jumps and noticing the direction of the jump. Move decimal in original number the same # of spaces and in the same direction. Example #1: (1) Look at the problem: 56 cm = _____ mm Look at the unit that has a number 56 cm On the device put your pencil on that unit. k km h hm d u d dam m dm c cm m mm Example #1: 2. Move to new unit, counting jumps and noticing the direction of the jump! Be careful NOT to count the spot you start from, where you put your pencil point. Only count the jumps! k km h hm d dam u m d c m dm cm mm One jump to the right! Example #1: 3. Move decimal in original number the same # of spaces and in the same direction. 56 cm = _____ mm 56.0. One jump to the right! Move decimal one jump to the right. Add a zero as a placeholder. Answer: 56cm = 560 mm Example #2: (1) Look at the problem. 7.25 L = ____ kL Look at the unit that has a number. 7.25 L On the device put your pencil on that unit. k h d kl hl dal u L d c m dl cl ml Example #2: 2. Move to new unit, counting jumps and noticing the direction of the jump! k h d u kL hL daL L d dL c m cL Three jumps to the left! mL Example #2: (3) Move decimal in original number the same # of spaces and in the same direction. 7.25 L = ____ kL .007.25 Move decimal to the left three jumps. Add two zeros as placeholders. Answer: 7.25 L = .00725 kL Three jumps to the left! Mass and Weight •Mass and weight are directly related as long as we remain on earth at the same elevation. That is, if one object has twice the mass of another, then its weight on earth would also be twice as large. Mass and Weight • Weight is force of the gravitational pull on an object (pounds, measured with a scale). It would be different on the moon than it is on earth. • Mass is a measure of the amount of matter in an object. (grams, measured with a BALANCE) What is a Liter?? Measuring Volume: The volume of a liquid is measured with a graduated cylinder. When liquid is poured into the cylinder, a curved surface called the meniscus is formed. Learning Check 1. 1000 m = 1 ___ a) mm b) km c) dm 2. ___ a) mg b) kg c) dg ___ a) mL b) cL c) dL a) mm b) cm c) dm 0.001 g = 1 3. 0.1 L = 1 4. 0.01 m = 1 ___ 1. 1000 m = 1 ___ 2. ___ 0.001 g = 1 3. 0.1 L = 1 ___ 4. 0.01 m = 1 ___ a) mm a) mg b) km c) dm b) kg c) dg a) mL b) cL c) dL a) mm b) cm c) dm Measured Numbers When you use a measuring tool is used to determine a quantity such as your height or weight, the numbers you obtain are called measured numbers. Exact Numbers Obtained when you count objects 2 soccer balls 1 watch 4 pizzas Obtained from a defined relationship 1 foot = 12 inches 1 meters = 100 cm Not obtained with measuring tools We will later learn that these numbers do not limit the number of significant figures reported. Learning Check Classify each of the following as an exact(e) or a measured (m) number. A.___Gold melts at 1064°C B.___1 yard = 3 feet C.___A red blood cell with diameter 6 x 10-4 cm D.___There were 6 hats on the shelf E.___A can of soda contains 355 mL of soda Solution Classify each of the following as an exact (e) or a measured(m) number. Give reason. A. m Requires a thermometer(measuring tool) B. e From a definition in U.S. system C. m Need measuring tool to determine D. e Counted the hats E. m Measured Two types of instruments used to take measurements. Digital display -measurement is displayed electronically by machine (Ex: electronic balance) Scaled instrument -instruments has numbered lines to determine measurement (Ex: ruler) REMEMBER TO INCLUDE THE DIGIT OF UNCERTAINTY IN YOUR MEASUREMENT. A digit that must be estimated is called uncertain. A measurement always has some degree of uncertainty. Record the certain digits and the first uncertain digit (the estimated number). Graduated cylinders shows each line (scale) represents 1 ml increments. This instrument is accurate to ones place, therefore estimated digit should be in tenth place. For scaled instruments the estimated digit must be determined by YOU. Liquid volume is 43.0 ml not 43 ml. The zero, in tenth place, is the estimated digit. Measurement of Volume Using a Buret The volume is read at the bottom of the liquid curve (meniscus). Meniscus of the liquid occurs at about 20.15 mL. Certain digits: 20.15 Uncertain digit: 20.15 Electronic thermometer is an example of a instrument that uses digital display. The last digit in these types of instruments is the estimated digit and is always supplied. The 6 in the tenth place in the estimated digit. 32.6 oC is the correct reading. All measurements have some degree of uncertainty. WHY????? When measurements are recorded CORRECTLY it must be written with a digit of uncertainty (estimated digit) and a unit. Last digit in ANY measurement is the digit of uncertainty (estimated digit). Precision and Accuracy Accuracy • Agreement of a particular value with the true value. Precision • Degree of agreement among several measurements of the same quantity. Measurement Accurate means "capable of providing a correct reading or measurement." In Chemistry: it means 'correct‘. How close you are to the accepted value. Measurement Accurate Measurement Precise X X X X X X Measurement Can you be neither accurate or precise? Measurement x This is a random like pattern, neither precise nor accurate. The darts are not clustered together and are not near the bull's eye. x x x x Measurement This is an accurate pattern, but not precise. The darts are not clustered, but their 'average' position is the center of the bull's eye. x x x x x Measurement This pattern is both precise and accurate. The darts are tightly clustered and their average position is the center of the bull's eye. x xxx x