energy in thermal system
advertisement
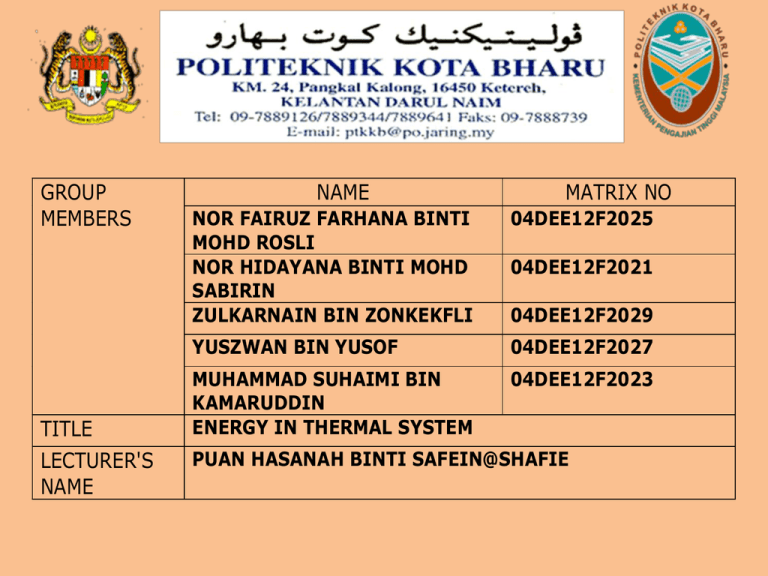
GROUP MEMBERS TITLE LECTURER'S NAME NAME MATRIX NO NOR FAIRUZ FARHANA BINTI MOHD ROSLI NOR HIDAYANA BINTI MOHD SABIRIN ZULKARNAIN BIN ZONKEKFLI 04DEE12F2025 YUSZWAN BIN YUSOF 04DEE12F2027 MUHAMMAD SUHAIMI BIN KAMARUDDIN ENERGY IN THERMAL SYSTEM 04DEE12F2023 04DEE12F2021 04DEE12F2029 PUAN HASANAH BINTI SAFEIN@SHAFIE (TO BE FILLED UP BY LECTURER) DATE OF SUBMISSION LECTURER'S COMMENTS RUBRIC ITEM 4 1. Procedures 2. 3. Participation Diagrams 4. Data 5. Calculation 6. Analysis 7. Error Analysis 8. Question 9. Conclusion 10. Subsmission Date TOTAL MARKS 3 2 1 ENERGY IN THERMAL SYSTEM OBJECTIVE To gain thermal equilibrium. To determine final temperature INFERENCE Thermal equilibrium is achieved when two substances reach the same temperature and exchange to heat energy. HYPOTHESIS The final temperature obtained from experiment will be the state in which the temperature of two substances reached the equal temperature at a certain time. 1. Mercury thermometer -1 2. Beaker -2 3. Bunsen burner or electric kettle -1 4. Electronic Balance/ Beam Balance -1 5. Hot hand protector -1 6. Tripod stand and wire gauze -1 7. Lighter gun -1 PROCEDURES 1. 2. 3. 4. 5. 6. 7. 8. Weigh the mass of an empty beaker and record as m1. Fill 200ml tap water into the beaker. Measure mass of the beaker with tap water and record as m2. Read the initial temperature of tap water and record as T1. Fill another empty beaker with 250ml water. Weigh the mass of the beaker and record as m3. Heat the beaker for 10 minutes. Read the temperature of the hot water Figure 1. Pour tap water into the beaker containing hot water gently. Stir the mixture slowly about 10 seconds. Record the final temperature as Tfinal. RESULT Table 1 Table 2 Mass, m Empty beaker, m1 Gram , g 204.8 Beaker with 200 ml cold water (tap water), Beaker with 250 ml hot water, m2 395.6 m3 443.5 Temperature, T °c Cold water (tap water), Hot water, T1 32 T2 96 The mixture, Tfinal 66 Use the values from the Tables 1 and 2 above to answer the following question: Mass of the cold water (tap water) mcold = m2 - m1 Mass of hot water mhot = m3 - m1 Mass of the mixture = mcold + mhot 190.8 g 238.7 g 429.5 g CALCULATION Calculate Tfinal by using the following equation:Tfinal = (mhot x Thot) + (mcold x Tcold) (mhot + mcold) Tfinal (from experiment) 66 Tfinal (from calculation) 67.57 % Error 2.324 TEMPERATURE LIGHTER GUN ELECTRONIC BALANCED HAND PROTECTOR ANALYSIS • Energy in thermal system or thermal equilibrium is achieved when two substances reach the same temperature and exchange to heat energy. • We calculate the gram(g) of the mass and celcius(°c) of the temperature, T. • The apparatus we using is mercury thermometer, beaker, Bunsen burner or electric kettle, electronic balance/beam balance, hot hand protector, tripod stand and wire gauze and lighter gun. ERROR ANALYSIS • Due to error in reading on the percentage error, we can find this error by using the Tfinal (from experiment) and Tfinal (from calculation). The formula of the Tfinal is Tfinal= (mhot x Thot) + (mcold x Tcold) (mhot + mcold) • We carry out the experiment twicely and we found that the reading is not same. QUESTION • Does the final temperature in this experiment is equal with the final temperature in calculation? No • If both of the temperatures are not the same, explain why and relate to thermal equilibrium principle? Because two objects at the thermal equilibrium have the same temperature and there is no net transfer of heat energy between the objects. CONCLUSION 1. After experiment, we should be able to gain thermal equilibrium. 2. We also can know how to determine final temperature. 3. As a student, we also can know how to find the result of the empty beaker, beaker with 200ml cold water (tap water), and beaker with 250ml hot water. 4. We also can found the temperature of the cold water (tap water), temperature of hot water and the temperature of the mixture of water. VIDEO EXPERIMENT











