Platinum Nanotubes Synthesized by Decomposition of Pt(acac)2 in
advertisement

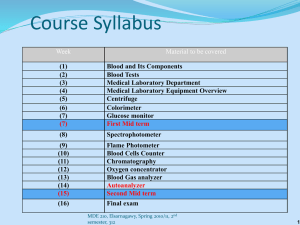
Leah Douglas1,3, Michael Bright1, Doug Aaron1, Alexander B. Papandrew1, and Thomas A. Zawodzinski, Jr.1,2 1Department of Chemical and Biomolecular Engineering, University of Tennessee, Knoxville, TN 2Materials Science and Technology Division, Oak Ridge National Laboratory, Oak Ridge, TN ³The University of Memphis, Memphis, TN OBJECTIVE The objective of this experiment was to increase electrode surface area and reduce the contact resistance between the membrane and the electrode. This was done by attaching a microporous electrode directly to the membrane. Variations of carbon inks, amounts of ink and types of carbon paper were considered in this effort. Flow batteries are a potential technology for energy storage because they allow for large amounts of energy to be stored and flexibility in size. Energy and power capacity can be independently scaled. One type of flow battery is the vanadium redox battery (VRB). An advantage of the VRB is that crossover only results in loss of charge, not cross contamination and all charge is stored in the electrolyte. MOTIVATION Cathode Anode 1.8 IR-FreePotential(V) Membrane Graphite Plate Gold Current Collector • Electrolyte: 2M vanadium in 5M SO⁴⁻ • Flow rate: 20mL/min • Potentiostat: Biologic HCP-803 up to 80 amps • Membrane: N117 for thickness • Carbon paper electrodes (SGL group) Electrode Mass 6.8 mg (5 layer), 17.4 mg (10 layer) Carbon:Ionomer 2:1 C:I, 50:1 C:I Carbon Paper 5% treated, untreated, none • 2:1 C:I composition • N117 membrane • No carbon paper present 1.6 1.4 1.2 1 0.8 0.6 2:1,5layer,ncp 2:1,10layer,ncp 0.4 0.2 0 0 50 100 150 200 Current(mA/cm²) 1.6 1.4 1.2 1 0.8 0.6 0.4 0.2 0 correct raw 0 100 200 300 400 Current flowing in electrochemical cells experiences intrinsic resistance (iR). To correct for this resistance, the high frequency resistance (HFR) is found Finding the areal specific resistance (ASR) allows for the calculation of an iRfree polarization curve. This is done by taking the HFR and multiplying it with the area of the membrane. Electrode: 5 layers, 50:1 C:I ink Electrode: 10 layers, 2:1 C:I ink 1.8 1.6 1.4 1.2 1 0.8 0.6 0.4 0.2 0 50:1 C:I 2:1 C:I 0 100 200 300 • 10 layer decal • 2:1 C:I composition • N117 membrane 1.6 1.4 1.2 1 0.8 0.6 0.4 Untreated carbon paper 5% PTFE No Carbon Paper 0.2 0 0 50 100 150 200 250 300 Current (mA/cm²) Different layers of ink were tested to see if, by adding more ink, performance would improve. Increased electrode surface area could result in better performance. However, the thicker electrode did not exhibit better performance. Potential (V) Cell Potential (V) Polarization curves allow for the determination of the dominant loss mechanisms in a battery. Activation loss is the result of slow reaction kinetics at low current. Ohmic loss is attributed to charge transport in the battery. Mass transport limitation is the inability to increase rate of electrolyte delivery to match increase in current. 1.8 IR-Free Potential (V) Setup Electrode Configuration 400 Current (mA/cm²) Different compositions of carbon and ionomer ink were considered. The goal here was to increase electrode surface area and improve the electrical conductivity of the electrode. Different types of carbon paper were tested to determine the effect on the battery. There was no discernable difference between the different carbon papers . Summary • Increased carbon content in the electrode resulted in improved performance. • No great performance improvement was observed when doubling the number of layers of ink used to make the electrodes. • Wet-proofed carbon paper performed similarly to non-wet-proofed carbon paper. • Lack of carbon paper resulted in noticeably greater activation loss. Acknowledments to NSF grant #1004083 for making this research possible.