Clinical Trial Data
advertisement
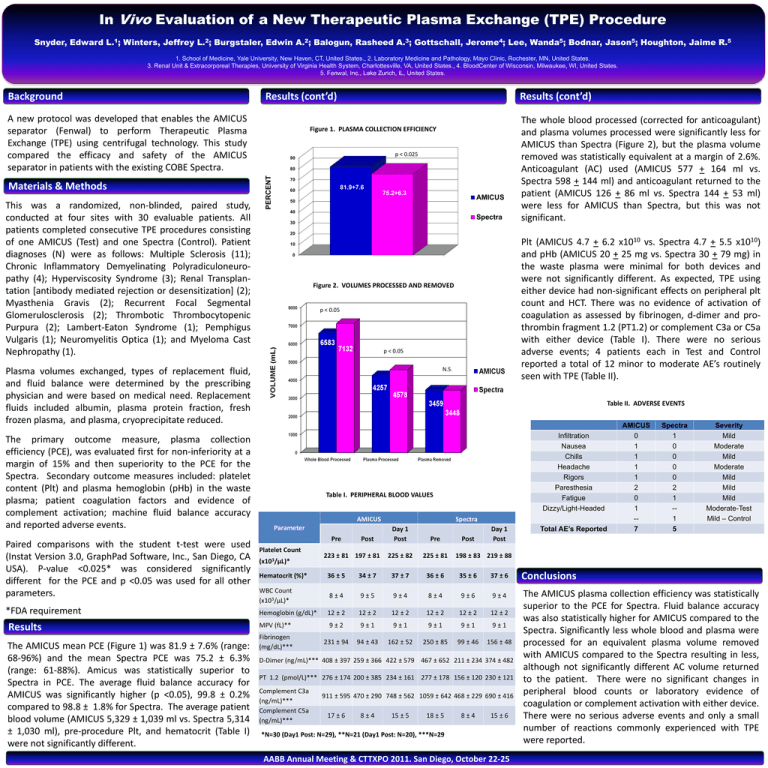
In Vivo Evaluation of a New Therapeutic Plasma Exchange (TPE) Procedure Snyder, Edward L.1; Winters, Jeffrey L.2; Burgstaler, Edwin A.2; Balogun, Rasheed A.3; Gottschall, Jerome4; Lee, Wanda5; Bodnar, Jason5; Houghton, Jaime R.5 1. School of Medicine, Yale University, New Haven, CT, United States., 2. Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN, United States. 3. Renal Unit & Extracorporeal Therapies, University of Virginia Health System, Charlottesville, VA, United States., 4. BloodCenter of Wisconsin, Milwaukee, WI, United States. 5. Fenwal, Inc., Lake Zurich, IL, United States. Background Results (cont’d) A new protocol was developed that enables the AMICUS separator (Fenwal) to perform Therapeutic Plasma Exchange (TPE) using centrifugal technology. This study compared the efficacy and safety of the AMICUS separator in patients with the existing COBE Spectra. This was a randomized, non-blinded, paired study, conducted at four sites with 30 evaluable patients. All patients completed consecutive TPE procedures consisting of one AMICUS (Test) and one Spectra (Control). Patient diagnoses (N) were as follows: Multiple Sclerosis (11); Chronic Inflammatory Demyelinating Polyradiculoneuropathy (4); Hyperviscosity Syndrome (3); Renal Transplantation [antibody mediated rejection or desensitization] (2); Myasthenia Gravis (2); Recurrent Focal Segmental Glomerulosclerosis (2); Thrombotic Thrombocytopenic Purpura (2); Lambert-Eaton Syndrome (1); Pemphigus Vulgaris (1); Neuromyelitis Optica (1); and Myeloma Cast Nephropathy (1). Figure 1. PLASMA COLLECTION EFFICIENCY p < 0.025 90 80 PERCENT Materials & Methods Results (cont’d) 70 81.9+7.6 60 75.2+6.3 AMICUS 50 40 Spectra 30 20 Paired comparisons with the student t-test were used (Instat Version 3.0, GraphPad Software, Inc., San Diego, CA USA). P-value <0.025* was considered significantly different for the PCE and p <0.05 was used for all other parameters. *FDA requirement Results The AMICUS mean PCE (Figure 1) was 81.9 ± 7.6% (range: 68-96%) and the mean Spectra PCE was 75.2 ± 6.3% (range: 61-88%). Amicus was statistically superior to Spectra in PCE. The average fluid balance accuracy for AMICUS was significantly higher (p <0.05), 99.8 ± 0.2% compared to 98.8 ± 1.8% for Spectra. The average patient blood volume (AMICUS 5,329 ± 1,039 ml vs. Spectra 5,314 ± 1,030 ml), pre-procedure Plt, and hematocrit (Table I) were not significantly different. Plt (AMICUS 4.7 + 6.2 x1010 vs. Spectra 4.7 + 5.5 x1010) and pHb (AMICUS 20 + 25 mg vs. Spectra 30 + 79 mg) in the waste plasma were minimal for both devices and were not significantly different. As expected, TPE using either device had non-significant effects on peripheral plt count and HCT. There was no evidence of activation of coagulation as assessed by fibrinogen, d-dimer and prothrombin fragment 1.2 (PT1.2) or complement C3a or C5a with either device (Table I). There were no serious adverse events; 4 patients each in Test and Control reported a total of 12 minor to moderate AE’s routinely seen with TPE (Table II). 10 0 Figure 2. VOLUMES PROCESSED AND REMOVED p < 0.05 p < 0.05 N.S. Plasma volumes exchanged, types of replacement fluid, and fluid balance were determined by the prescribing physician and were based on medical need. Replacement fluids included albumin, plasma protein fraction, fresh frozen plasma, and plasma, cryoprecipitate reduced. The primary outcome measure, plasma collection efficiency (PCE), was evaluated first for non-inferiority at a margin of 15% and then superiority to the PCE for the Spectra. Secondary outcome measures included: platelet content (Plt) and plasma hemoglobin (pHb) in the waste plasma; patient coagulation factors and evidence of complement activation; machine fluid balance accuracy and reported adverse events. The whole blood processed (corrected for anticoagulant) and plasma volumes processed were significantly less for AMICUS than Spectra (Figure 2), but the plasma volume removed was statistically equivalent at a margin of 2.6%. Anticoagulant (AC) used (AMICUS 577 + 164 ml vs. Spectra 598 + 144 ml) and anticoagulant returned to the patient (AMICUS 126 + 86 ml vs. Spectra 144 + 53 ml) were less for AMICUS than Spectra, but this was not significant. Table II. ADVERSE EVENTS Infiltration Nausea Chills Headache Rigors Paresthesia Fatigue Dizzy/Light-Headed Table I. PERIPHERAL BLOOD VALUES AMICUS Parameter Pre Platelet Count (x103/µL)* Post 223 ± 81 197 ± 81 Spectra Day 1 Post Pre 225 ± 82 225 ± 81 Post Day 1 Post Total AE’s Reported AMICUS 0 1 1 1 1 2 0 1 -7 Spectra 1 0 0 0 0 2 1 -1 5 Severity Mild Moderate Mild Moderate Mild Mild Mild Moderate-Test Mild – Control 198 ± 83 219 ± 88 Hematocrit (%)* 36 ± 5 34 ± 7 37 ± 7 36 ± 6 35 ± 6 37 ± 6 Conclusions WBC Count (x103/µL)* 8±4 9±5 9±4 8±4 9±6 9±4 Hemoglobin (g/dL)* 12 ± 2 12 ± 2 12 ± 2 12 ± 2 12 ± 2 12 ± 2 MPV (fL)** 9±2 9±1 9±1 9±1 9±1 9±1 Fibrinogen (mg/dL)*** 231 ± 94 94 ± 43 162 ± 52 250 ± 85 99 ± 46 156 ± 48 The AMICUS plasma collection efficiency was statistically superior to the PCE for Spectra. Fluid balance accuracy was also statistically higher for AMICUS compared to the Spectra. Significantly less whole blood and plasma were processed for an equivalent plasma volume removed with AMICUS compared to the Spectra resulting in less, although not significantly different AC volume returned to the patient. There were no significant changes in peripheral blood counts or laboratory evidence of coagulation or complement activation with either device. There were no serious adverse events and only a small number of reactions commonly experienced with TPE were reported. D-Dimer (ng/mL)*** 408 ± 397 259 ± 366 422 ± 579 467 ± 652 211 ± 234 374 ± 482 PT 1.2 (pmol/L)*** 276 ± 174 200 ± 385 234 ± 161 277 ± 178 156 ± 120 230 ± 121 Complement C3a (ng/mL)*** Complement C5a (ng/mL)*** 911 ± 595 470 ± 290 748 ± 562 1059 ± 642 468 ± 229 690 ± 416 17 ± 6 8±4 15 ± 5 18 ± 5 8±4 15 ± 6 *N=30 (Day1 Post: N=29), **N=21 (Day1 Post: N=20), ***N=29 AABB Annual Meeting & CTTXPO 2011. San Diego, October 22-25