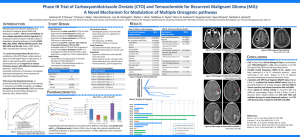
2012 AACR TRC102 + Temodar Phase 1b poster
advertisement

First in human phase 1 clinical trial of the base-excision repair inhibitor Methoxyamine-HCl (TRC102; MX) given as an one-hour intravenous infusion in combination with Temozolomide (TMZ) in patients with solid tumors. Panos S. Savvides, Yan Xu, Lili Liu, Lisa Rogers, Joseph Bokar, Paula Silverman, Afshin Dowlati, Stanton L. Gerson Seidman Cancer Center and CASE Comprehensive Cancer Center, Cleveland, OH. Patient Characteristics TMZ ↓ MX ↔ Cycle 2 and subsequent (q 4 weeks) Week1 Day1 Day2 Day3 Day4 Day5 TMZ ↓ MX ↔ ↓ ↓ ↓ -1 1 2 3 4 5 Dose level 1 Dose level 2 Primary tumors N= 13 N= 4 Lung, breast, pancreatic, colon, esophageal, ovarian, oropharyngeal, salivary gland, skin adnexal tumor Dose level -1 Dose level 1 Primary tumors 15 15 30 60 100 150 Eligibility Criteria ●Histologically confirmed solid tumor; incurable ●Priorchemotherapy and/or radiation are allowed. ●Prior temozolomide treatment is not restricted. ●Age >18 years. ●ECOG performance status <2 ●Life expectancy > 12 weeks. ●Patients must have normal organ and marrow function 2.5 1DLT (grade 3 psychosis) Note: Expansion to 10 evaluable patients b/o 1 idiosyncratic event (gr 3 allergic reaction) with no DLTs Dose level 2 No DLTs Cohort B (CNS disease) Dose level -1 Dose level 1 No DLTs No DLTs 1.5 2 1.0 1.5 1 0.5 0.5 2.0 2h 0h 4h 24h 0h 2h 4h 24h AP sites formed over time after a single dose of TMZ and low levels of AP sites were detected in cells treated with combination of TMZ and MX. Treatment Toxicities Dose level 1 MX-bound AP site TMZ+MX 0 N=3 N=3 Brain, pancreatic, head and neck Cohort A ( No CNS disease) 2.0 TMZ Typical PK profile: TRC102 15mg/m2, (Pt #13, cycle#1) Comet Assay, Neutral conditions (Detect DNA double strand breaks) A 100 150 150 150 150 150 3 TRC102 half-life:55.04 h (range:12.2-100.3h, n=20) Cohort B (CNS disease) ↓ DOSE-ESCALATION SCHEDULE (cohort A) Dose TMZ MX Dose Level (mg/m2/day) x5days (mg/m2) Level Level Level Level Level Level Cohort A ( No CNS disease) Comet Assay, Alkaline conditions (Detect DNA single strand breaks) B 4 4 Tail length (arbitrary units) Cycle 1 (duration: 2 weeks) Week1 Day1 Day2 Day3 Day4 Day5 Correlative studies AP sites relative to control Background: MX is the first base excision repair (BER) inhibitor evaluated in humans. MX blocks the BER pathway by covalently binding to apurinic/pymidinic (AP) sites in DNA. In several preclinical studies, improved therapeutic efficacy has been demonstrated with various chemotherapeutic agents. The final results of the phase I clinical trial of the combination of pemetrexed and oral MX have been published (GJ Weiss et al). Initial results and correlative studies of the alkylating agent TMZ and MX were previously presented (AACR Annual Meeting 2009, abstract#5433). As initial PK analyses on patient samples revealed a distinct PK profile in humans, with a 10-fold increase in estimated half-life when compared to the half-life observed in dogs, the protocol has been amended with MX administration adjusted from a 5-day intravenous continuous infusion to a 1-hour intravenous infusion. Methods: This ongoing phase I dose-escalation trial investigates the safety, pharmacokinetic (PK) and pharmacodynamic (PD) profile of MX given as an one-hour intravenous infusion in combination with TMZ. PD markers, including analysis of AP sites measured on DNA extracted from patients’ mononuclear cells (PBMCs) as well as DNA strand break determined by comet assay at multiple time points during the 5-day treatment, are included. Results: 23 patients have enrolled, at dose-levels (DL) 1 and 2 (TMZ 150 mg/m2/day, days 1-5 and MX 15 mg/m2 and 30 mg/m2 respectively) in two cohorts. In cohort A (patients with no-CNS disease, n =13 at DL1 and n=4 at DL2) primary tumors include lung, breast, pancreatic, colon, esophageal, ovarian, oropharyngeal, salivary gland, skin adnexal tumors. In cohort B (patients with CNS disease, n =3 at each DL1 and 2) primary tumors include brain, pancreatic, head and neck tumors.The combination of MX and TMZ has been well-tolerated. For the non-CNS involvement cohort, one DLT was observed at DL1; grade 3 psychosis in a patient with progressive disease on increasing doses of opioids. A grade 3 allergic reaction classified as an idiosyncratic event resulted in further expansion of the cohort to 10 evaluable patients with no additional DLTs observed. No DLTs have been observed at DL2. For the CNSinvolved cohort no DLTs have been observed to date. Three patients had stable disease (lung, ovarian, head and neck primaries) Conclusions: MX has a distinct PK profile in humans, which has allowed us to move to a convenient one-hour infusion regimen for further development. Ongoing data from the 1hour infusion MX regimen in the first in humans MX phase I clinical trial demonstrate that MX in combination with TMZ is well tolerated. No significant anemia (the DLT observed in the MX and pemetrexed trial) has been observed at DL1 and 2 to date; possibly because of lower MX dose administered at DLs 1 and 2 compared to MX DLT dose in the pemetrexed trial. Trial remains open to accrual. Schema Tail length (arbitrary units) Abstract TMZ 3 TMZ+MX 2 1 TMZ 3 TMZ+MX 2 1 0 0 0 2 4 Time (hr) 24 0 2 4 24 Time (hr) DNA strand breaks detected by Comet assay in PBMCs in patients treated with TMZ and MX. (A) DNA double strand breaks detected by neutral comet assay. (B) DNA single strand breaks detected by alkaline comet assay. The cells were mixed with low-melting agarose, lysed and the DNA was electrophoresed in both alkaline and neutral conditions to detect DNA single and double strand breaks, respectively. Conclusion Best Response ● Stable disease ( n = 3) ● Treatment duration 7, 10, 11 months ● Primary tumors (lung, ovarian, head & neck) ● TRC102 can be safely administered as a one-hour infusion ● The combination of TRC102 with TMZ is well tolerated ● Correlative studies demonstrate that TRC102 interrupts base excision repair in all treated patients, even at the lowest level Supported in part by NIH grant: R21 CA126149 ClinicalTrials.gov Identifier: NCT00892385