Thermochemistry I
advertisement
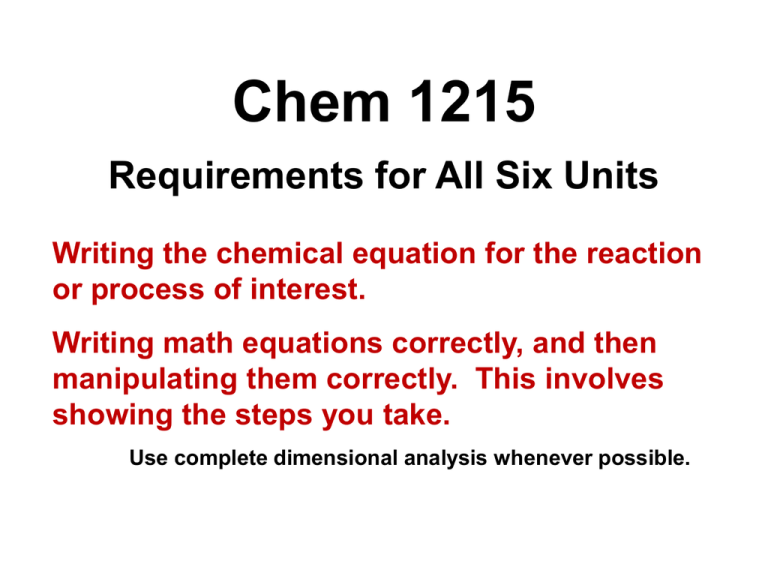
Chem 1215 Requirements for All Six Units Writing the chemical equation for the reaction or process of interest. Writing math equations correctly, and then manipulating them correctly. This involves showing the steps you take. Use complete dimensional analysis whenever possible. UNIT 1 Thermochemistry Thermochemistry Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat. Energy comes in two forms: potential energy (P.E.) – stored energy chemical, electrostatic, positional kinetic energy (K.E.) – energy of motion Temperature is a measure of the kinetic energy of atoms and molecules. System and Surroundings Thermodynamics is the study of energy and its transformations. To apply thermodynamics to our study of thermochemistry, we have to be very careful about what we call the system and what we call the surroundings. What we single out for study is the system. Everything else is the surroundings. The universe is the sum of the system and the surroundings. Closed Systems vs. Open Systems Closed systems are the most straightforward to study. They can exchange energy but not matter with their surroundings. open system closed system Open systems can exchange both energy and matter with their surroundings. Internal Energy Energy is the capacity to do work or to transfer heat. The internal energy (E) is the sum of all the kinetic and potential energies of all the components of a system. E can be difficult to measure. ΔE, the change in internal energy, can be measured. ΔE = Efinal – Einitial FYI: internal energy = translational + vibrational + rotational + electronic + chemical potential energies ΔE: Work and Heat A closed system exchanges energy (ΔE) with its surroundings through work and heat. 1. Work is energy used to move an object against a force. work = force x distance d after expansion w = Fd Closed system performing work on the surroundings: expanding gas moves a piston. ΔE: Work and Heat 1. Work is energy used to move an object against a force. work = force x distance w = Fd There are several types of work. • Pressure-volume: w = -PΔV • Electrical: w = -nFE • Elongation: w = fl • Surface expansion: γσ Work and Heat Closed systems exchange energy with their surroundings in the form of work and heat. 1. Work is energy used to move an object against a force. 2. Heat is the energy transferred from a hotter object to a colder one as the result of a temperature difference. Work and heat are what bring about changes in the internal energy (ΔE). First Law of Thermodynamics The energy of the universe does not change. ΔEuniv = 0 This is the 1st Law as it applies to the universe. Note the “univ” subscript. It says that energy changes in the system and the surroundings must balance. First Law of Thermodynamics Another way to state the first law is in terms of the system we are studying. The system and the surroundings can exchange heat q and mechanical energy (work) w. w>0 q>0 • A positive value for heat means heat goes into the system. • A positive value for work means work is done on the system. First Law of Thermodynamics as it Applies to a System If the energy of the universe does not change, that means the change in the energy of the system, which is the change in internal energy ΔE, must be equal to the heat that goes into the system and the work done on the system. ΔE = q + w Note the lack of subscripts. No subscripts means we are referring to the system. This is the 1st Law as it applies to a system. The 1st Law of Thermodynamics: ΔE = q + w The signs of thermodynamic variables are as important as their values: ΔE > 0 means the internal energy increased. q > 0 means heat was transferred into the system. w > 0 means work was done on the system. w>0 q>0 ΔE Calculating ΔE ΔE = q + w EXAMPLE: H2(g) and O2(g) are placed in a cylinder/piston arrangement and ignited. The gases in the system expand against the piston and lose 1150 J of heat to the surroundings. The joule (J) is the SI unit for energy. In terms of SI base units, 1J=𝟏 𝒌𝒈 𝒎𝟐 𝒔𝟐 An older unit of energy is the calorie: 1 cal = 4.184 J q = -1150 J Calculating ΔE ΔE = q + w EXAMPLE: H2(g) and O2(g) are placed in a cylinder/piston arrangement and ignited. The gases in the system expand against the piston and lose 1150 J of heat to the surroundings. The expanding gases from the reaction cause the piston to rise, doing 480 J of work against the atmosphere. w = - 480 J ΔE = q + w = -1150 - 480 = -1630 J q = -1150 J The internal energy decreased, was evolved. and heat Exothermic Processes A process in which the system evolves heat is exothermic. The reaction between H2(g) and O2(g) to form H2O(l) is exothermic. 2H2(g) + O2(g) 2H2O(l) + heat Einitial = internal energy of the reactants, H2(g) and O2(g) Efinal = internal energy of the product, H2O(l) Internal Energy and Energy Diagrams What does ΔE<0 mean? That the internal energy of the products is lower than the internal energy of the reactants. Internal Energy and Energy Diagrams What does ΔE<0 mean? ΔE < 0, and ΔE = Eprod – Ereac,* so Eprod – Ereac <0, or Eprod <Ereac It means that the internal energy of the products is lower than the internal energy of the reactants. * Is ΔE = Eprod – Ereac the 1st Law? Calculating ΔE ΔE = q + w EXAMPLE: Calculate the change in the internal energy ΔE for a process in which the system absorbs 141 J of heat from the surroundings and does 85 J of work on the surroundings. ΔE = q + w = 141 - 85 ΔE = 56 J What does ΔE>0 mean? 1. Efinal > Einitial , or 2. The internal energy E increased. In this example, heat was absorbed by the system. w = - 85 J q = 141 J Endothermic Processes A process in which the system absorbs heat is called endothermic. The physical process that occurs when a cold pack is activated is endothermic: NH4NO3(s) + H2O(l) + heat NH4NO3(aq) The elephant and the piston A large cylinder/piston arrangement filled with air at 1 bar and 25°C was just sitting there, minding its own business, when an elephant stepped on it, compressing the air inside to 36% of its original volume and raising the pressure to 9.1 bar, which equated to 14365 J of work. It happened so fast that no heat could enter or leave the cylinder, resulting in a T rise to 715°C. What is the value of the change in internal energy of the system? Was the process endothermic or exothermic? Can you use Kinetic-Molecular Theory to explain what’s happening? The elephant and the piston A large cylinder/piston arrangement filled with air at 1 bar and 25°C was just sitting there, minding its own business, when an elephant stepped on it, compressing the air inside to 36% of its original volume and raising the pressure to 9.1 bar, which equated to 14365 J of work. It happened so fast that no heat could enter or leave the cylinder, resulting in a T rise to 715°C. What is the value of the change in internal energy of the system? ∆E = q + w = 0 + 14365 J = 14365 J The elephant and the piston A large cylinder/piston arrangement filled with air at 1 bar and 25°C was just sitting there, minding its own business, when an elephant stepped on it, compressing the air inside to 36% of its original volume and raising the pressure to 9.1 bar, which equated to 14365 J of work. It happened so fast that no heat could enter or leave the cylinder, resulting in a T rise to 715°C. Was the process endothermic or exothermic? The gas got hotter, right? So…exothermic, right? NOT! q = 0 The process was neither. This type of process is called adiabatic. The elephant and the piston Can you use Kinetic-Molecular Theory to explain what’s happening? The pressure increase comes from the decrease in volume and the consequent increase in collisions with the wall. The temperature rise is due to the fact that work was done on the system, causing the internal energy to increase. For an ideal gas, KE = 3/2 RT. If KE is increased (from the work done on the system) and there is no loss from heat, then the temperature must rise.