Pediatric epilepsy syndromes 2014 - Stefanie Jean
advertisement

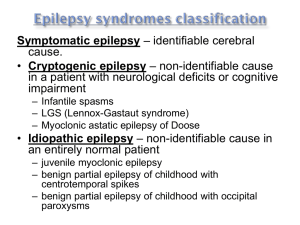
Pediatric Epilepsy Syndromes Stefanie Jean-Baptiste Berry, MD Pediatric Neurologist/Epileptologist Northeast Regional Epilepsy Group Introduction Most Recent ILAE Definition April 2014: (1) At least two unprovoked (or reflex) seizures occurring >24 h apart (2) One unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (at least 60%) after two unprovoked seizures, occurring over the next 10 years (3) Diagnosis of an epilepsy syndrome Introduction Term Epilepsy Syndrome has been used by ILAE to refer to “a complex of signs and symptoms that define a unique epileptic condition.” Epilepsy syndromes denote specific constellations of clinical seizure type(s), EEG findings, other characteristic clinical features such as age at onset, course of epilepsy, associated neurologic and neuropsychological findings, and underlying pathophysiologic or genetic mechanisms. Introduction Identification of a specific syndrome is important to define the best treatment and accurately prognosticate long-term outcome. Idiopathic (primary) – presumed etiology is genetic Symptomatic (secondary) types – underlying etiology is known or presumed based on other evidence of brain dysfunction, such as developmental delay. Generalized seizures vs. Localization-related seizures. Benign Familial Neonatal Seizures Typically present during the first few weeks of life Focal, multifocal, or generalized seizures Seizures are brief but occur 20 – 30 times per day Seizures may be difficult to control Benign Familial Neonatal Seizures Normal neurologic exam No specific EEG features Family history of similar neonatal seizures is important for diagnosis Autosomal dominant with 85% penetrance Linked to voltage gate potassium channels KCNQ2 and KCNQ3 on chromosomes 20q and 8q Benign Familial Neonatal Seizures Outcome generally favorable Resolution of seizures typically in early to midinfancy Normal neurodevelopment 8-16% of patients will later develop epilepsy as adults Benign Idiopathic Neonatal Seizures Healthy, neurologically normal term neonates Seizures typically begin on the fifth day of life or “fifth day fits” Partial clonic seizures that migrate, increase in frequency and culminate in status epilepticus Benign Idiopathic Neonatal Seizures No specific EEG features There is no family history of seizures Seizures typically resolve after 24 hours Children have normal neurodevelopment No increased risk of seizure recurrence Generalized (Genetic) Epilepsy with Febrile Seizures Plus Characterized by febrile and afebrile seizures Febrile seizures continue beyond the typical age of remittance, 6 years Afebrile seizures are infrequent, brief, and include generalized tonic-clonic, myoclonic, complex partial and atonic seizures. Generalized (Genetic) Epilepsy with Febrile Seizures Plus Seizures start 4 months and 10 years, with mean onset of 2 years Prior to onset of afebrile seizures, GEFS+ can be difficult to distinguish from febrile seizure Careful family history is important Generalized (Genetic) Epilepsy with Febrile Seizures Plus Interictal EEG may be normal or demonstrate generalized epileptiform discharges Genetically heterogenous autosomal dominant with 60-80% penetrance Within families multiple phenotypes with variable severity exist Generalized (Genetic) Epilepsy with Febrile Seizures Plus Multiple gene mutations linked to GEFS+ including 19q13.1 (SCN1B gene), 2q2324.2 (SCN1A gene) and 5q31.1-33.1 Excellent prognosis in most children Seizures typically spontaneously remit by age 11 years Up to 30% may have more severe epilepsy Myoclonic Astatic Epilepsy of Doose Rare, affecting 1:10,000 Presents in previously neurologically healthy preschool-aged children Multiple seizure types including generalized tonic clonic, myoclonic, absence, atonic, myoclonic, myoclonic atonic Myoclonic atonic seizures most prominent Myoclonic Astatic Epilepsy of Doose Outcome and course are variable – complete remission to intractable epilepsy with poor cognitive outcome EEG demonstrates 2-3Hz spike and wave discharges Up to 32% of children have a family history of epilepsy Inheritance pattern is unknown Childhood Absence Epilepsy Comprises 2-15% of childhood epilepsy Onset is usually 4 and 10 years of age Girls 2-5 times more likely to have absence Most patients with childhood absence have normal neurological exams and normal intelligence scores Childhood Absence Epilepsy Inherited in autosomal dominant pattern with incomplete penetrance chromosomes 20q, 16p13.3, and 8q24.3 Caused by abnormalities in T-type calcium channels, which are responsible for rhythmic depolarizing activity in the thalamic neurons Childhood Absence Epilepsy Generalized type of seizure Characterized by sudden discontinuation of activity with loss of awareness, responsiveness, and memory, with an abrupt recovery Automatisms, brief clonic jerks, and loss of postural tone can also be seen Childhood Absence Epilepsy Usually lasts 5 to 10 seconds Up to hundreds of seizures per day Seizures can be provoked by hyperventilation in approximately 90% of children Childhood Absence Epilepsy Classic EEG finding in typical absence seizures is the sudden onset of 3-Hz generalized symmetrical spike and wave complexes Interictal EEG background is normal Childhood Absence Epilepsy Normal EEG Childhood Absence Epilepsy Absence Seizure Childhood Absence Epilepsy Most children only experience absence seizures 3% will experience generalized tonicclonic seizures Childhood Absence Epilepsy With typical absence seizures, if consistent EEG, normal intelligence, and normal neuro exam, no further work up is necessary Primary drugs of choice are ethosuximide (Zarontin), valproic acid (Depakote), and lamotrogine (Lamictal) Most clinicians start with ethosuximide (T-type calcium channel blocker) because of fewer incidence of side effects Childhood Absence Epilepsy Valproic acid is the drug of choice in patients with both absence and generalized tonic-clonic seizures Lamictal is a safer choice for female teens Duration of therapy is variable, although general rule is to taper off therapy after 2 seizure free years Childhood Absence Epilepsy Absence status epilepticus is characterized by sustained impairment of consciousness associated with generalized 3-Hz spike and wave Patients often exhibit facial twitching, eye blinking, staring and automatisms Treatment is usually with IV lorazepam or Depakote (not Dilantin) Childhood Absence Epilepsy Children with early onset (mean age 6) have the best prognosis with complete remission 2 to 6 years after onset Onset of absence seizures before age 3 years is associated with increased likelihood of neurodevelopmental abnormalities Childhood Absence Epilepsy Average age of cessation is 10 years old Typical absence seizures generally have a favorable prognosis with remission rates of approximately 80 percent Can precede juvenile myoclonic epilepsy in 11-18% of cases Childhood Absence Epilepsy Risk factors for intractability: myoclonic or atonic component to seizure, generalized tonic clonic seizures occur at onset and photosensitivity on EEG Juvenile Absence Epilepsy Onset is typically between ages 10 and 16 Clinical seizures similar to CAE Seizures occur less frequently and may be longer duration More likely to experience generalized tonic clonic seizures Interictal EEG, 3.5-4Hz spike and polyspike and wave Response to treatment good, but may be lifelong Juvenile Myoclonic Epilepsy Affects 4 to 10% of all patients with epilepsy and up to 26% of patients with idiopathic generalized epilepsy Seizures typically present between 12 and 18 years Inheritance is complex Classic form is likely autosomal dominant and inherited and linked to 6p12-11 Juvenile Myoclonic Epilepsy Commonly 1st seizure noted is a GTC in setting of sleep deprivation Careful questioning will reveal a history of myoclonic seizures and possible absence seizures in the preceding months Occurs in both genders with equal frequency Juvenile Myoclonic Epilepsy Seizure types include generalized tonicclonic, myoclonic, and absence 100% have myoclonic seizures, 96% have GTC, and only 20% with absence Generalized tonic-clonic and myoclonic seizures tend to occur in morning upon awakening Seizures are precipitated by sleep deprivation, alcohol ingestion and in women, menstruation Juvenile Myoclonic Epilepsy Myoclonic seizures are brief and bilateral, flexor jerks of the arms, which may be repetitive Jerks sometimes affect the legs, causing the patient to fall Consciousness is not impaired during myoclonic seizures Juvenile Myoclonic Epilepsy Delays in diagnosis are common, often until a generalized tonic-clonic seizure brings the child to medical attention Interictal EEG in JME consists of generalized spike and polyspike-andwave discharges of 4 to 6 Hz, usually maximal in the frontocentral regions Photic stimulation often provokes a discharge (30 to 90%) Juvenile Myoclonic Epilepsy Generalized Polyspike and Wave Discharge Juvenile Myoclonic Epilepsy Traditional treatment was valproic acid with 85%-90% response but many side effects Newer effective drugs include levetiracetam, lamotrogine, topiramate and zonisamide Carbamazepine, phenytoin and gabapentin may exacerbate seizures Response to treatment is excellent but treatment is lifelong Benign Childhood Epilepsy with Centrotemporal Spikes (BCECTS) Most common form of idiopathic partial epilepsy Accounts for 13%-23% of all childhood epilepsies Although it is clearly familial, its mode of inheritance is unclear Onset is between 4 and 10 years Peak age of onset is 7-8 years Children are neurologically and cognitively normal Benign Childhood Epilepsy with Centrotemporal Spikes (BCECTS) Nocturnal seizure, usually occurring after falling asleep or before awakening is typical Seizures described as unilateral paresthesias of the face, unilateral clonic or tonic activity involving the face, speech arrest, drooling with preserved consciousness Can have secondarily generalized tonicclonic seizures Benign Childhood Epilepsy with Centrotemporal Spikes (BCECTS) EEG background is normal Spikes are present at the midtemporal and central (centrotemporal) head region Marked activation of spikes in drowsiness and sleep is characteristic, and 30% of cases show spikes only during sleep Benign Childhood Epilepsy with Centrotemporal Spikes (BCECTS) EEG Findings BCECTS EEG Findings BCECTS Benign Childhood Epilepsy with Centrotemporal Spikes (BCECTS) If typical history, normal neuro exam, and characteristic EEG findings, an MRI is not necessary No treatment is necessary in patients with infrequent, nocturnal, partial seizures If seizures are frequent (20%)and disturbing to patient and family, treatment with Tegretol or Trileptal is usually successful Benign Childhood Epilepsy with Centrotemporal Spikes (BCECTS) Excellent prognosis Spontaneous remission occurs by age 15 to 17, often much earlier Some children may develop language, cognitive or behavioral deficits which improve after remission Panayiotopoulos Syndrome Early-onset benign childhood epilepsy with occipital paroxysms Average age of 3-6 years Normal development Characterized by nocturnal seizures in two-thirds, with tonic eye deviation, vomiting (autonomic) and impaired consciousness Hemiconvulsions and GTC seizures are common Panayiotopoulos Syndrome Interictal EEG shows normal background with high-amplitude occipital spike-wave complexes on eye closure Seizures are infrequent and one third of patients will only have a single seizure Paradoxically, seizures are frequently prolonged (status epilepticus) Excellent prognosis –seizures usually cease within 2 years onset Treatment with medication is not necessary Late-Onset Childhood Epilepsy with Occipital Paroxysms - Gastaut Syndrome Rare condition Ages 6-12 years, average 8 years 21-37% of cases have family history of epilepsy. Late-Onset Childhood Epilepsy with Occipital Paroxysms - Gastaut Syndrome Seizures characterized by visual hallucinations or ictal blindness often with gaze deviation or eyelid fluttering Often followed by postictal headache Focal seizures often evolve into hemiconvulsions or GTC seizures Seizures more frequent but shorter duration Late-Onset Childhood Epilepsy with Occipital Paroxysms - Gastaut Syndrome EEG –normal background, interictal occipital high amplitude spike wave with attenuation on eye opening Ictal recordings show fast occipital spikes EEG Findings Childhood Epilepsy with Occipital Paroxysms Late-Onset Childhood Epilepsy with Occipital Paroxysms - Gastaut Syndrome Brain MRI is normal (neuroimaging is recommended) Seizures respond well to carbamazepine 50-60% have seizure remission within 2-4 years Infantile Spasms Occurs almost exclusively in infants younger than 1 year of age Incidence is 1 in 4,000 to 6,000 births Spasm onset is usually within the first 4-8 months of life Familial occurrence is rare Infantile Spasms Seizures typically described as brief, symmetrical contractions of the neck trunk, and extremities Flexor or extensor muscles can be involved Asymmetrical infantile spasms are rare Spasms frequently occur immediately upon, or soon after, arousal 80% of spasms occur in clusters Infantile Spasms Interictal EEG is characterized hypsarrhythmia defined as high voltage slow waves and multifocal spikes In a small number of infants, the background activity may appear normal Most common ictal pattern is characterized by generalized slow wave transient followed by attenuation of background activity Infantile Spasms Hypsarrhythmia Infantile Spasms Etiologies of infantile spasms can be determined in 60%-90% of cases Tuberous sclerosis is a major cause of infantile spasms (up to 25%) Other etiologies include: lissencephaly, Sturge-Weber, HIE, meningitis, and inborn errors of metabolism A minority of infants have idiopathic infantile spasms Infantile Spasms First line is ACTH but has many side effects Vigabatrin, Prednisone, and Topamax are also effective drugs A challenge with a 100mg of IV pyridoxine should be considered in idiopathic cases Infantile Spasms Overall prognosis is poor, with only 5% of the total patient population having normal outcomes Severe or very severe impairment was observed in 67% of patients Only factor that seems to effect longterm outcome is whether the patient is classified as cryptogenic (unknown) or symptomatic Lennox Gastaut Syndrome Classic 1. 2. 3. Triad: Multiple seizure types (tonic, atonic, and atypical absence) EEG pattern of slow spike and wave discharges Cognitive Impairment Lennox Gastaut Syndrome Onset usually between 1 and 8 years Most cases between 2 and 5 years Onset rare after 10 years Males > females 10% of childhood epilepsies Lennox Gastaut Syndrome Neurological symptoms may be absent initially 9-39% preceded by infantile spasms High seizure frequency At least 1 episode of status in 50%-75% Lennox Gastaut Syndrome No single cause 2/3 symptomatic (cortical dysplasias, TS, metabolic, prenatal/perinatal) Cryptogenic (unknown cause) LGS with normal brain MRI accounts for 1/3 cases Lennox Gastaut Syndrome Tonic 1. 2. 3. 4. Seizures: Most characteristic – prerequisite Flexor movement of the head and trunk with apnea preceded by brief cry (axial) Abduction, elevation of limbs, usually arms with clenching of the fists (axorhizomelic) Sustained contraction involving most muscles, including distal (global) Lennox Gastaut Syndrome Atypical 1. 2. 3. Absence: Second most common type Brief loss/lapse of consciousness Difficult to identify Lennox Gastaut Syndrome Atonic Seizures: 1. “Drop attacks” 2. Particularly hazardous 3. 56% of patients Other types: 1. Focal 2. GTC 3. Unilateral Clonic Lennox Gastaut Syndrome EEG 1. 2. 3. 4. 5. Findings in LGS: Slow background Diffuse slow-spike and wave, 1.5 - 2.5Hz Paroxysmal Fast Activity Focal or multifocal discharges in 14%18% 75% of electroclinical persists to adult Lennox Gastaut Syndrome Slow Spike and Wave Lennox Gastaut Syndrome Paroxysmal Fast Activity Lennox Gastaut Syndrome Seizures resistant to therapy Drugs used in combination, mostly guided by anecdotal evidence or personal experience AED may help control one seizure type while worsening another First line AEDs are Valproic Acid and Benzodiazepines Rufinamide and Felbamate Lennox Gastaut Syndrome Overall, unfavorable prognosis Worse prognosis: 1. Symptomatic 2. Infantile spasms 3. Early onset seizures 4. Higher seizure frequency 5. Constant background slowing Dravet Syndrome Rare Incidence of 1 in 20,000 to 40,000 Approximately 80% of cases have mutation in SCN1A (sodium channel) Vast majority of mutations are sporadic Close relatives have a higher rate of seizures (GEFS+) Dravet Syndrome Begins in the first year of life in previously well infant Generalized or focal clonic seizure is often prolonged Onset frequently triggered by fever, infection, vaccination or warm bath Progression to further recurrent prolonged focal seizures with and without fever Dravet Syndrome End of second year through age 5, myoclonic jerks appear Atypical absence and complex partial seizures with autonomic symptoms occur in 50% in preschool years Tonic seizures are rare Dravet Syndrome Initial EEG is typically normal Over time, background slows Multifocal and generalized polyspike and wave discharges appear Epileptiform discharges activated by drowsiness and photic stimulation MRI normal early, later shows atrophy Dravet Syndrome Infants are developmentally normal at onset Regression or lack of progression is seen between 1 and 4 years, stabilization after at lower level Visuomotor skills more affected than language Dravet Syndrome Seizures are medically refractory Valproic Acid and clobazam are first line Second line AEDs include topiramate, levetiracetam and zonisamide Lamotrigine and carbamazepine should be avoided – can aggravate seizures Mortality rate 16-18% (status, SUDEP, drowning) References Wirrell, Elaine and Nickels, Katherine C. Pediatric Epilepsy Syndromes. Continuum Lifelong Learning Neurology 2010; 16(3) 57-85. Ebersole and Pedley, Current Practice Of Clinical Electroencephalography. Third Edition 2003. Menke, Sarnat and Maria. Child Neurology. Seventh Edition. 2006 Pellock, Dodson and Bourgeois. Pediatric Epilepsy: Diagnosis and Therapy. Second Edition. 2001