Heme emergencies
advertisement

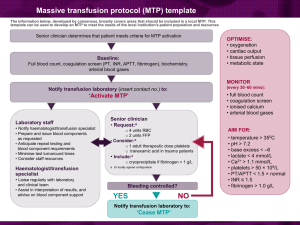
EVALUATION OF THE BLEEDING PATIENT HISTORY (inherited or acquired bleeding tendency?) • Prior invasive procedures, dental extractions • Family history • Medications, alcohol PHYSICAL EXAM • Mucosal/skin vs soft tissue bleeding? • Bleeding from one or multiple sites? • Mucosal hemangiomas, skin/joint laxity, etc Platelet defects and vessel disorders: immediate bleeding from skin and mucosal surfaces, petechiae Coagulation factor deficiency: delayed bleeding into soft tissues Bleeding confined to an operative site is usually due to a severed vessel Initial laboratory evaluation • • • • • Platelet count PT/INR aPTT Fibrinogen Platelet function screen Prothrombin time/INR Long PT/INR – Liver disease – Vitamin K deficiency – Warfarin or warfarin analogs (rat poison) – DIC – Inherited conditions rare – Won’t detect hemophilia, factor VIII inhibitor, heparin at therapeutic concentrations Magnitude of test abnormality usually proportional to clinical severity aPTT Long aPTT – Heparin (therapeutic or contaminant) – Hemophilia A or B – von Willebrand disease (low VIII – but PTT may be normal) – Factor XI deficiency – Factor VIII inhibitor – Contact factor deficiency (do not cause bleeding) – Lupus anticoagulant (does not cause bleeding) – Less sensitive than PT/INR to liver disease, DIC, warfarin Magnitude of test abnormality not necessarily proportional to clinical severity Platelet function screen • Replaces the bleeding time • Advantages – Ex vivo test (no skin incision) – Better standardized – Better sensitivity and specificity PFA-100 Platelet function screen Sensitive to: Defective platelet adhesion in von Willebrand disease Platelet dysfunction due to many drugs Inherited platelet disorders Not useful in patients with moderate or severe Thrombocytopenia Rarely the only abnormal test in an acute bleeding disorder Conditions that may cause bleeding with normal or near-normal screening tests • Mild hemophilia (factor level 20-30% of normal) • Von Willebrand disease • Factor XIII deficiency (very rare) • Fibrinolytic disorders • Vascular disorders (Ehlers-Danlos, amyloid, etc) ACUTE THROMBOCYTOPENIA ACUTE THROMBOCYTOPENIA Immune-mediated platelet consumption • ITP: Platelets < 10K common, but major bleeding unusual. Rarely develops during hospitalization • Drug-induced purpura: Platelets < 10K common, higher bleeding risk than ITP. Look for recent changes in drug regimen, quinine exposure • Heparin-induced thrombocytopenia: Up to 50% develop thrombosis. Usually not bleeding May occur after heparin d/c’d. Platelets rarely < 20 K. • Post-transfusion purpura: Recent blood transfusion, multiparous woman, sudden severe drop in platelet count (usually <10K). DRUGS MOST LIKELY TO CAUSE THROMBOCYTOPENIA * * * * * * Hematology 2009;153 ACUTE THROMBOCYTOPENIA Other causes • TTP: Systemic signs and symptoms, high LDH, schistocytes on blood smear. Platelets may be < 10K. Rarely develops during hospitalization • HUS, other microangiopathies: Systemic signs and symptoms, renal failure, high LDH, schistocytes on blood smear. Platelets rarely <10K • DIC: Acute systemic illness, abnormal clotting times, platelets rarely <10K • Marrow failure: Other cell lines abnormal. Rarely presents with isolated severe thrombocytopenia ACUTE THROMBOCYTOPENIA Plts <10K? Bleeding the predominant symptom? Onset during hospitalization? ITP Drug-induced thrombocytopenia TTP Post transfusion purpura Marrow failure (if other counts low) ITP Drug-induced thrombocytopenia Post transfusion purpura DIC DIC Heparin-induced thrombocytopenia Post-transfusion purpura Drug-induced thrombocytopenia ACUTE SEVERE THROMBOCYTOPENIA History • Recent transfusions, previous pregnancies (posttransfusion purpura) • Recent drug exposure • Neuro symptoms (TTP) • Fever, other systemic symptoms (DIC, TTP) • Autoimmune disease, CLL (ITP) ACUTE SEVERE THROMBOCYTOPENIA Blood smear • • • • Platelet clumping (pseudothrombocytopenia) RBC fragments/schistocytes (TTP, DIC) Abnormal WBC (marrow dyscrasia) Large platelets suggest rapid production ACUTE THROMBOCYTOPENIA Further diagnostic testing Drug-dependent antibody panel Heparin antibody test HPA-1a antibody testing (post-transfusion purpura) D-dimer, INR, fibrin monomer (DIC) LDH, urinalysis, retic count (TTP) ADAMTS-13 activity (TTP) Marrow biopsy often unnecessary (for isolated thrombocytopenia) • ITP is diagnosis of exclusion • New onset thrombocytopenia in hospitalized patient is unlikely to represent ITP or TTP ACUTE THROMBOCYTOPENIA Treatment CONDITION TREATMENT ITP Steroids, IVIG, splenectomy Drug-induced purpura Stop drug! IVIG/steroids? Heparin-induced thrombocytopenia TTP Stop all heparin; alternative anticoagulant (eg argatroban) Plasma exchange, immunosuppression No platelet transfusions; washed RBC, IVIG Post-transfusion purpura Marrow failure transfusions www.ouhsc.edu/platelets HEMOPHILIA HEMOPHILIA • Inherited deficiency of factor VIII (hemophilia A) or factor IX (hemophilia B) • Sex-linked inheritance; almost all patients male • Most bleeding into joints, muscles; mucosal and CNS bleeding uncommon • Severity inversely proportional to factor level: < 1%: severe, bleeding after minimal injury 1-5%: moderate, bleeding after mild injury > 5%: mild, bleeding after significant trauma or surgery (may not be diagnosed until adulthood) HEMOPHILIA Treatment of bleeding episodes • Unexplained pain in a hemophilia should be considered due to bleeding unless proven otherwise • External signs of bleeding may be absent • Treatment: factor replacement, pain control – Most adult patients self-administer factor • Test for inhibitor (antibody vs factor) if unexpectedly low response to factor replacement – Most inhibitors occur in children TREATMENT OF BLEEDS IN HEMOPHILIA • Administer appropriate clotting factor concentrate – 20-40 U/kg for minor bleeds, repeat daily for 2-3 days – 40-50 U/kg for major bleeds, repeat daily for 4-7 days – 50 U/kg q 12 hours for life-threatening bleeds • 1 U/kg should increase plasma level of factor by about 2% – Initial dose somewhat higher with factor IX concentrate (greater volume of distribution, but longer half-life) TREATMENT OF HEMOPHILIACS WITH INHIBITORS • • • • Call a hematologist Recombinant factor VIIa High dose factor VIII (if low titer inhibitor) Induce tolerance with daily factor infusion ± immunosuppression ACQUIRED FACTOR VIII DEFICIENCY • Autoantibody to factor VIII (most common autoimmune factor deficiency) • Most patients elderly • Often presents with severe soft tissue or mucosal bleeding (different bleeding pattern than inherited hemophilia) • Laboratory: prolonged aPTT not corrected by mixing with normal plasma, factor VIII activity typically < 10% – Bleeding risk not proportional to factor level – Normal INR and platelet count • Treatment: rVIIa, immunosuppression DISSEMINATED INTRAVASCULAR COAGULATION DISSEMINATED INTRAVASCULAR COAGULATION • Rapid formation & lysis of intravascular fibrin • Consumption of clotting factors, platelets, inhibitors • Life-threatening underlying disease • Bleeding due to uncontrolled fibrinolysis, thrombocytopenia, clotting factor consumption and tissue injury from underlying disease • Tissue injury/necrosis due to microvascular occlusion, hypotension, cytokine-mediated endothelial damage • Most deaths due to underlying disease PUPURA FULMINANS Pneumococcal sepsis in splenectomized patient NEJM 2001;344:1593 DIAGNOSIS OF DIC 1. Underlying disease capable of causing DIC? 2. Evidence of accelerated clotting factor and platelet consumption, and increased fibrinolysis? If both present DIC is likely SCREENING FOR DIC • • • • • D-dimer (most sensitive) PT/INR Fibrinogen CBC/platelet count Fibrin monomer (most specific) TREATMENT OF DIC • TREAT UNDERLYING DISEASE! • Clotting factor & inhibitor replacement for patients with significant bleeding: Fresh frozen plasma (goal INR ≤ 1.6) Cryoprecipitate (goal fibrinogen ≥ 100) Platelets (goal platelet count 50-75K) • Pharmacologic inhibitors (selected pts with refractory bleeding) Heparin (low dose) Antifibrinolytics (Amicar, tranexamic acid) THROMBOTIC THROMBOCYTOPENIC PURPURA (TTP) TTP • Microangiopathic hemolytic anemia • Thrombocytopenia • Organ dysfunction (CNS, renal, other) due to small vessel occlusion • Untreated mortality rate >90% • With treatment mortality < 20% • 1-2 cases/yr @ UWHC TTP • Caused by autoimmune destruction of ADAMTS-13 protease that modulates von Willebrand factor multimer size • Very large multimers clump platelets • Microthrombi damage RBC and block vessels TTP • Thrombocytopenia (may be severe) • Hemolytic anemia (high LDH, low haptoglobin, schistocytes in blood smear) • Organ dysfunction – Neurologic symptoms – Renal dysfunction (hematuria, proteinuria) – Cardiac (arrhythmia) • ADAMTS-13 activity low (usually <5%) TTP - DDX • • • • • • • Cancer (may be occult) Pregnancy complications (HELLP, etc) Hemolytic-uremic syndrome Vasculitis (SLE, scleroderma) HIV infection DIC Drugs (calcineurin inhibitors, mitomycin C, interferon, clopidogrel, ticlopidine) TTP • Urgent plasma exchange – Plasma infusion if PE not immediately available • Immunosuppression – Corticosteroids – Rituximab for patients with resistant, refractory or relapsed disease • Do not transfuse platelets unless there is lifethreatening bleeding BLOOD PRODUCTS AND DRUGS IN THE TREATMENT OF BLEEDING DISORDERS BLOOD COMPONENT TRANSFUSION TO TREAT OR PREVENT BLEEDING • Platelets: – Active bleeding: if platelet count < 50K – Prophylaxis: when platelets <10-20K – Patient having surgery: if platelets < 50-100K • FFP (active bleeding): – When INR > 1.6 • Cryoprecipitate (active bleeding): – Goal fibrinogen level 100 DDAVP (Desmopressin) • Vasopressin analogue, stimulates VWF/factor VIII release from endothelium • Intravenous or intranasal administration • Increases plasma VWF/factor VIII levels for 18-24h, enhances platelet adhesion • Effective in – Type I VWD (usually not in type 2, never in type 3) – Mild hemophilia A – Platelet dysfunction (variably effective) • Side effects usually mild (HA, flushing, hyponatremia with repeated dosing) AMICAR (epsilon aminocaproic acid) • Antifibrinolytic: inhibits plasmin formation and binding to fibrin • Uses – Treating DIC with hyperfibrinolysis, or bleeding after thrombolytic drugs – Prophylaxis in severe thrombocytopenia – GI tract bleeding – Menorrhagia – Prophylaxis after dental extraction in hemophilia • Short half-life, needs frequent dosing (4-24 grams/d) • Oral or intravenous administration • Alternative: tranexamic acid (longer half-life) Recombinant Factor VIIa (rFVIIa) • Augments tissue factor-induced coagulation • FDA approved for treatment of Factor VIII inhibitor w/bleeding • Off-label use is common, efficacy unclear: – Other coagulation inhibitors (including drugs) w/bleeding – Emergency reversal of coagulopathy (eg, warfarin) in patients with lifethreatening bleeding • Potent procoagulant → risk of thrombosis • Very expensive! Prothrombin complex concentrate (PCC) • Mixture of vitamin K dependent procoagulant factors: II, IX, X ,VII • Used to rapidly correct warfarin effect (or severe vitamin K deficiency) in acutely bleeding patient or prior to urgent surgical procedure • Advantages vs FFP: – Less volume – No risk of TRALI, low risk of allergic rxn – Less risk of virus transmission VITAMIN K Indications: 1. Correction of vitamin K deficiency 2. Treatment of warfarin or superwarfarin overdose 3. Prophylaxis in patients at risk for vitamin K deficiency Oral or IV administration (unreliable absorption with subcutaneous injection) ANTICOAGULANT-RELATED BLEEDING Bleeding events/100 patient-yr BLEEDING RISK VS INR 200 150 100 50 0 <2 2.0-2.9 3-4.4 INR 4.5-6.9 >7 Lancet 1996; 348:423 Treatment of warfarin-induced coagulopathy in the non-bleeding patient Dentali et al, J Thrombos Haemost 2006;4:1853 Treatment of warfarin-induced coagulopathy in the bleeding patient Dentali et al, J Thrombos Haemost 2006;4:1853 Reversal of heparin and LMWH • Heparin: – FFP won’t help – Protamine sulfate: 1 mg/100U heparin – Neutralizes 80% of heparin • LMWH – FFP won’t help – Protamine neutralizes 40% of LMWH New anticoagulant drugs: Dabigatran, rivaroxaban, apixaban • • • • • • If PT and aPTT normal overdose is unlikely No specific antidote Activated charcoal if drug taken recently PCC ~ 50 U/kg (rivaroxaban and apixaban) Consider dialysis (dabigatran only) FFP likely to be ineffective, risk of volume overload, TRALI TRANSFUSION REACTIONS IMMEDIATE HEMOLYTIC TRANSFUSION REACTION • • • Intravascular lysis of transfused rbcs by complement, IgM Causes: Transfusion of ABO-incompatible blood Transfusion of ABO-incompatible plasma Non-ABO antibodies Clinical manifestations: Fever (but most febrile reactions not hemolytic) Back pain Dark or red urine (hemoglobinuria) Bronchospasm Shock DIC Organ failure (esp kidneys) Death IMMEDIATE HEMOLYTIC TRANSFUSION REACTION Evaluation of suspected cases • • • Check blood product/paperwork to ensure correct product given Notify blood bank/transfusion service Obtain blood and urine samples: Plasma and urine hemoglobin Direct Coombs test Repeat crossmatch/antibody screen Repeat ABO/Rh typing IMMEDIATE HEMOLYTIC TRANSFUSION REACTION Management • Stop transfusion immediately • IV crystalloid or colloid • Maintain BP, heart rate • Maintain airway • Diuresis fluid, • loop diuretic (mannitol may cause volume overload) Monitor renal and coagulation status DELAYED HEMOLYTIC TRANSFUSION REACTION • IgG-mediated lysis of transfused red cells (usually extravascular, non-ABO) • Usually begins 5-10 days after transfusion • Jaundice, falling Hct, positive direct Coombs test, fever • Not generally life-threatening FEBRILE, NONHEMOLYTIC TRANSFUSION REACTION • Cause: cytokines released by leukocytes during • • • • storage; antibodies to HLA antigens on transfused or donor PMNS Incidence: 0.5% of units transfused More common in multiply transfused recipients Fever, chills, respiratory distress in severe reactions Reduced incidence/severity with leukocyte-poor product TRANSFUSION-RELATED ACUTE LUNG INJURY (TRALI) • Hypoxemia with bilateral pulmonary infiltrates • No increase in central venous or pulmonary artery • • • pressures Usually begins acutely within 6 hours of transfusion Clinical: acute respiratory distress, fever, chills Pathophysiology: 1. Underlying lung injury (eg, sepsis, pneumonia) causes PMNs to adhere to pulmonary capillaries 2. Mediators in transfused blood product (neutrophil antibodies, cytokines) activate PMNs with resultant capillary injury TRANSFUSION-RELATED ACUTE LUNG INJURY TRANSFUSION-RELATED ACUTE LUNG INJURY (TRALI) • Risk: FFP > platelets > RBC • Treatment: stop transfusion (if still in progress); oxygen; ventilatory support if necessary; pulse corticosteroids OTHER ACUTE NON-INFECTIOUS COMPLICATIONS OF TRANSFUSION • Allergic reactions • Anaphylaxis (IgA-deficient recipient) • Lung damage from microaggregates (massive transfusion) • Transfusion-associated circulatory overload (“TACO”) • Bacterial infection (mainly with platelet transfusion) • Hypothermia (rapid infusion of refrigerated blood) • Citrate toxicity/hypocalcemia (massive transfusion or apheresis) • Graft-vs-host disease • Air embolism Transfusion-related deaths 2005-2010 TRALI TACO HTR (non-ABO) HTR (ABO) Bacterial Infection Anaphylaxis 2005 29 1 16 6 8 0 2006 35 8 9 3 7 1 2007 34 5 2 3 6 2 2008 16 3 7 10 7 3 2009 13 12 8 4 6 1 2010 18 8 2 2 2 4 • TRALI – Transfusion-associated lung injury • TACO – Transfusion-associated circulatory overload • HTR – Hemolytic transfusion reaction