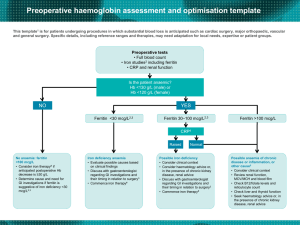
Deferasirox > 30 mg/kg/d
advertisement
Managing Iron Overload in Beta Thalassemia Major: Focus on Cardiac Iron Ali Taher, MD American University of Beirut Lebanon Baseline Patient Characteristics • At presentation – 22-year-old male patient diagnosed with beta thalassemia major at age 6 months – Normal ECG – Echocardiography showed LVEF of 70% – No history of hepatitis B – Hepatitis C-positive by PCR • Received peg-interferon and ribavirin from March 2003 until March 2004, after which PCR was negative ECG = electrocardiogram; LVEF = left ventricular ejection fraction; PCR = polymerase chain reaction Treatment History: Iron Chelation Therapy • Patient transfused for 21 years (since 1983): total of 14 blood transfusions/year Time Period Chelation Regimen 1984-2001 DFO 35-45 mg/kg/d 5x/wk 2001-2004 DFP 100 mg/kg/d (clinical trial) 2004 to mid-2005 DFO 35 mg/kg/d 5x/wk (clinical trial ended) • Serum ferritin range: 1823-4350 µg/L • Received calcium and folic acid supplements • Patient expressed dissatisfaction with burdensome subcutaneous regimen – Was often noncompliant with treatment DFO = deferoxamine; DFP = deferiprone Oral Chelators: Potential to Improve Compliance Oral Chelator Dosing Schedule Toxicity Profile Deferiprone Thrice daily Neutropenia Agranulocytosis Deferasirox Once daily Nausea, diarrhea ● ESCALATOR Study (N=237): Compared pt ratings for satisfaction and convenience with prior tx (DFO or DFP) vs deferasirox1 Prior Therapy Deferasirox (baseline) (end of study) Satisfied or very satisfied with therapy 23% 91% Therapy convenient or very convenient 22% 93% Time lost to therapy for daily activities (mean ± SD, hrs/month) 30.1 ± 44.2 3.2 ± 8.6 1. Taher A, et al. Acta Haematologica. 2010;123:220-225. Deferasirox Therapy • Patient was willing to switch to deferasirox – In year prior to starting deferasirox, patient received 14 transfusions, each 2 units PRBC (9530 mL total) 2.3 units PRBC/mo Baseline Measurement Serum ferritin LIC by MRI Cardiac T2* by MRI Serum Cr and ALT 3560 µg/L 12.4 mg Fe/g dry wt 10.6 ms Within normal range ALT = alanine aminotransferase; Cr = creatinine; LIC = liver iron concentration; MRI = magnetic resonance imaging; PRBC = packed red blood cells Iron Overload Assessment Increased risk of complications Increased risk of cardiac disease Iron-Overloaded State Parameter Normal Mild Moderate Severe LIC, mg Fe/g dry wt < 1.2 3–7 >7 > 15 Serum ferritin μg/L < 300 Transferrin saturation, % 20–50 > 1000 to < 2500 > 2500 > 50 T2*, ms > 20 14–20 8–14 <8 Alanine aminotransferase, U/L < 250 > 250 Labile iron pool, μM 0–0.4 > 0.4 Patient has moderate-to-severe iron overload serum ferritin is 3560 µg/L; LIC = 12.4 mg Fe/g dry wt; cardiac T2*= 10.6 ms Jensen PD, et al. Blood. 2003;101:4632-4639. Data from Jensen PD, et al. Blood. 2003;101:91-96. Olivieri NF, Brittenham GM. Blood. 1997;89:739-761. Deferasirox Dosing by Transfusion Requirements and Therapeutic Goals Recommended initial deferasirox dosage 20 mg/kg/d Starting dosages may also be modified as follows: Transfusion requirement Therapeutic goal Deferasirox dosage PRBCs > 14 mL/kg/mo (~4 adult units) Reduction of body iron 30 mg/kg/d PRBCs < 7 mL/kg/mo (~2 adult units) Maintenance of body iron 10 mg/kg/d For patients well managed on DFO, suggested starting dosage may be numerically half DFO dosage, eg: DFO 40 mg/kg/d 5 d/wk EXJADE® (deferasirox) Basic Prescribing Information. Novartis Pharma AG. National Prescribing Information should be followed. Deferasirox 20 mg/kg/d Serum Ferritin (μg/L) Serum Ferritin After 7 Mo Deferasirox 20 mg/kg/d Deferasirox 20 mg/kg/d Months Case Study Details: Response to Dosage Increase • Patient’s dosage increased to 30 mg/kg/d • Dosage further increased to 35 mg/kg/d after 4 months because serum ferritin level was relatively unchanged • Patient continued to receive 2.3 units PRBC/month Treatment and Assessments: Serum Ferritin Over 2 Years Serum Ferritin (μg/L) 6,000 Serum ferritin levels decreased to 389 μg/L 5,000 4,000 3,000 2,000 1,000 0 DFX 20 Apr- Jun- Aug05 05 05 DFX 20 = deferasirox 20 mg/kg/day DFX 30 = deferasirox 30 mg/kg/day DFX 35 = deferasirox 35 mg/kg/day DFX 30 Oct05 Dec05 DFX 35 Feb06 Apr06 Months Jun06 Aug06 Oct06 Dec06 Feb07 Apr07 Treatment and Assessments: Serum Creatinine and ALT Over 2 Years Creatinine (µmol/L)/ALT (U/L) Serum Cr ULN Serum Cr > 33% above baseline ALT ULN DFX 20 DFX 30 DFX 35 Months Cardiac T2* (ms)/LIC (mg Fe/g dry wt) Improvement in Cardiac T2* and LIC Over 2 Years of Therapy After 2 years: Cardiac T2* improved by 60% LIC improved by 85% DFX 20 April 2005 DFX 30 DFX 35 April 2006 April 2007 Successful Chelation Achieved Via Titration • Although patient received deferasirox 20 mg/kg/d for almost 7 months, serum ferritin levels remained stable • Dosage was increased to 30 mg/kg/d for 4 months and then to 35 mg/kg/d • Patient did not experience any progressive increases in serum creatinine or liver enzyme levels Variable Serum ferritin LIC Cardiac T2* Result After 2 Years Deferasirox Treatment Decreased to 389 µg/L Normalized to 1.3 mg Fe/g dry wt Improved to 17 ms Deferasirox > 30 mg/kg/d: Safety Most common drug-related adverse events, as assessed by investigators (observed in > 1 patient after dose escalation to > 30 mg/kg/d) Frequency, n (%) Adverse event Median exposure (weeks) Before dose escalation After dose escalation 115.4 36.1 ALT increase 12 (5.4) 7 (3.1) Vomiting 17 (7.6) 6 (2.7) Abdominal pain 15 (6.7) 3 (1.3) 3 (1.3) 3 (1.3) Nausea 24 (10.7) 3 (1.3) Serum creatinine increase 13 (5.8) 3 (1.3) Rash 19 (8.5) 2 (0.9) Diarrhea 12 (5.4) 2 (0.9) Abdominal pain (upper) Taher A, et al. Br J Haematol. 2009;147:752-759. Follow-Up • At this time, deferasirox treatment was stopped, because serum ferritin levels were < 500 µg/L at 2 consecutive study visits – Deferasirox dosage lowered to 0 mg/kg/d as of 18 May 2007 and later reinitiated when serum ferritin rose to > 1000 µg/L Follow-Up: Serum Ferritin < 500 µg/L at 2 Consecutive Visits • Prescribing information suggests temporary discontinuation of deferasirox when serum ferritin levels drop to < 500 µg/L • However, patient still had – Continuous transfusion requirement and cardiac iron overload (cardiac T2* = 17 ms) – No evidence of iron chelator-related toxicity • Consider decreasing dose when serum ferritin levels drop to < 1000 µg/L; titrate to 500 µg/L instead of discontinuing treatment Safety Profile in Patients Who Achieved Serum Ferritin Levels ≤ 1000 μg/L ● In total, 163 patients (25.0%) achieved serum ferritin levels ≤ 1000 μg/L after a median of 1.2 years on deferasirox ● Most common drug-related adverse events were transient and mild to moderate in severity Drug-related adverse event Number of pts (%) Nausea 25 (15.3%) Diarrhea 17 (10.4%) Vomiting 11 (6.7%) Abdominal pain 10 (6.1%) Skin rash 9 (5.5%) ● 10 pts (6.1%) had 2 consecutive serum creatinine increases of > 33% above baseline and ULN; most were only marginally > ULN and none were > 2x ULN – All increases were nonprogressive and responded promptly to dose reduction ULN = upper limit of normal Porter J, et al. Poster presented at ASH 2007 [poster 986]. Successful Chelation Achieved: Key Lessons • Deferasirox effectively removes iron from the blood and organs • Deferasirox at 30-40 mg/kg/d is effective in patients with liver and cardiac iron overload – Adjustments should be made in steps of 5 or 10 mg/kg/d and should be tailored to individual patient response and therapeutic goals (maintenance or reduction of iron burden) • Careful dose titration is necessary to avoid overchelation; however, treatment should not be interrupted based on serum ferritin values alone