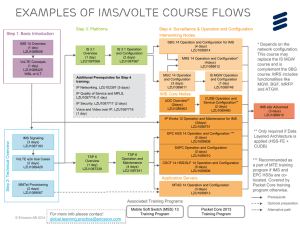
AANS/CNS Figures to CTAF Thrombectomy Coverage Review
advertisement

Figures for Draft Response to IMS III, MR RESCUE, and SYNTHSESIS Trials Figure 1: Lay Press Judgment May Belie a Deeper Examination of the Data. Truman ultimately defeated Dewey for the Presidency Subject enrollment by CTA and treatment group All Subjects n=656 Baseline CTA/MRA n=306 47% No Baseline CTA/MRA n=350 292 Baseline CTAs and 14 baseline MRAs Figure 2: Number of IMS III Patients who had pre-procedural imaging. This group represents the key subset analysis because of confirmed Large Vessel Occlusion (LVO) IMS III: No Treatable Occlusion by CTA • 80 Subjects: No treatable occlusion by operator - 41: M3 and/or M4 at Angiography by Core Lab - 15 CTA - 1 No Occlusion - 14 Occlusion - 1 ICA Terminus - 8 M1 - 4 M2 - 1 M3 Figure 3: Over 80 patients in IAT arm of IMS III had no treatable occlusion on angiography (Nearly 20%) Endovascular therapy not administered n=89 Reason Frequency No treatable arterial occlusion (operator) 80* Inability to access 3 Recanalization after baseline angiogram 2 Occlusion, but not responsible lesion 2 No reason 2 Figure 4: Over 20% of IAT cohort had no endovascular therapy administered 90-Day Modified Rankin Scale by Baseline NIHSS Strata and Treatment in IMS III NIHSS 8-19 IV tPA Endovascular only MRS <=2 (%) Risk Difference NIHSS ≥ 20 Endovascular 146 (48.3) 74 (49.3) 31 (23.5) -0.01 (-0.11, 0.09) IV tPA only All Endovascular IV tPA only 12 (16.7 177 (40.8) 86 (38.7) 0.07 (-0.04, 0.18) 0.02 (-0.06, 0.09) CMH p-value 0.70 Breslow Day p-value 0.27 Figure 5: Higher NIHSS corresponded with much greater endovascular treatment likely due to the increased incidence of LVO in subjects with NIHSS > 20 and greater opportunity to realize benefit Figure 6: Recent High Quality Trials Demonstrate: Endovascular is Efficacious (pre-specified analysis) Endovascular confers a statistically significant benefit across the spectrum of mRS A. Demchuk, IMS III: Comparison of Outcomes between IV and IV/IA Treatment in Baseline CTA Confirmed ICA, M1, M2 and Basilar Occlusions, slide 20, Presented at ISC 2013, Honolulu Hawaii With CTA-confirmed occlusion at baseline, representative of current practice, IMS III has a statistically significant positive outcome for endovascular 7 Figure 7: Recent High Quality Trials Demonstrate: Endovascular is Safe. In IMS III, despite reduced dose IV tPA and being subjected to angiography (with 20% of pts without occlusion), endovascular therapy had NO increase in death or symptomatic ICH IMS III Primary Safety End Points Endovascular Therapy (n=434) Death at 90 days - no. (%) 83 (19.1%) Symptomatic ICH at 30 hours – 27 (6.2%) no. (%) Intravenous tPA Alone (n=222) 48 (21.6%) 13 (5.9%) p value 0.52 0.83 8 Figure 8: Recent High Quality Trials Demonstrate: Endovascular is Safe. SYNTHESIS Primary Safety End Points Endovascula Intravenou p r Therapy s tPA Alone value (n=181) (n=181) Death at 7 days - no. (%) 14 (8%) Symptomatic ICH at 7 days – no. 10 (6%) (%) 11 (6%) 10 (6%) 0.53 0.99 SYNTHESIS conclusion: Subjecting ALL potential IV tPA patients to IA therapy, including those with minimal deficit (NIHSS of 2 included) and without confirmation of occlusion, demonstrated EQUAL efficacy to IV tPA with NO significant safety concerns 9 Figure 9: Percentage of Patients who achieved a functional outcome in IMS III based on reperfusion result (p=0.001) In IMS III, independent functional outcome (mRS 0-2) was strongly associated with TICI 2b3 revascularization. Though TICI 2b-3 is the modern endovascular standard, a low percentage of patients in IMS III achieved this technical result due to older, inferior technologies. TICI 2a was considered a good outcome in IMS III but clearly does not translate into good clinical outcomes Angiographic Reperfusion in IMS III N TICI 2-3 (%) TICI 2b-3 (%) Internal Carotid Artery 65 65 38 M1 of Middle Cerebral Artery 135 81 44 M2 if Middle Cerebral Artery Multiple M2s Basilar 61 22 4 70 77 NA 44 23 NA Figure 10: Few patients in IMS III met the clinically significant reperfusion standard due to use of first generation technologies. These TICI 2b and 3 rates fall well short of modern SWIFT and TREVO series IMS III TICI Reperfusion Primary Target Occlusion Primary Target Vessel All ICA extracranial occlusion ICA Intracranial M1 Percent with Percent with TICI 2-3 at TICI 2b-3 at Frequency completion completion of procedure of procedure 328 6 65 74% 83% 65% 40% 33% 38% ICA-T (41) (63%) (36%) Other Intracranial ICA (24) (67%) (42%) 135 81% 44% Figure 11: Low TICI 2b-3 rates due to first generation endovascular technologies persisted in IMS III independent of LVO location. Half of reperfusion successes in IMS III were only TICI 2a results. Embolectomy, Standard Case, Embolectomy, Standard Case Study Outcomes penumbral penumbral nonpenumbral nonpenumbral (n=34) (n=34) (n=30) (n=20) Score on 90 day modified Rankin Score Unadjusted Mean 3.9 3.4 4 4.4 Median 4 3 4 4 95% CI 3.3 to 4.4 2.8 to 4.0 3.4 to 4.6 3.6 to 5.2 Adjusted Mean 3.3 3.4 4 4.2 Median 4 3 4 4 95% CI 3.2 to 4.4 2.9 to 3.9 3.8 to 4.7 1.7 to 4.8 p 0.23 0.3 Good Outcome at 90 days # of patients (%) Adjusted analysis (%) Death; number (%) Hemorrhage; number (%) Symptomatic Asymptomatic Final infarct Volume No. of patient evacuated Median (interquartile range) - mL Absolute infarct growth No of patients evacuated Median - mL 7 (21) 14 6 (18) 9 (26) 23 7 (21) 5 (17) 9 6 (20) 2 (10) 10 6 (30) 0.48 0.39 0.75 3 (9) 19 (56) 3 (6) 14 (41) 0 23 (77) 0 12 (60) 0.24 0.04 32 32 30 19 58.1 (34.5 to 138.2) 37.3 (24.9 to 172.6 (84.6 to 217.1 (144.3 to 78.3) 273.8) 282.8) 32 32 10 19 27.1 6.7 55.1 83.8 <0.001 0.009 Figure 12: Due to poor technical performan ce of first generation devices, IAT patients in MR RESCUE did NOT achieve greater reperfusio n than Standard Medical Care Patients Figure 13: Those MR RESCUE patients that DID achieve reperfusion enjoyed better clinical outcomes Patients with Reperfusion or Recvascularization Pationes without reperfusion or Revascularization 43 43 Mean score on 90-day modified Rankin scale (95% CI) 3.2 (2.6 to 3.8) 4.1 (3.7 to 4.5) 0.04 Mean absolute infarct growth (interquartile range - mL) 9.0 (-13.7 to 50.3) 72.5 (5.6 to 120.7) <0.001 79 22 Mean score on 90-day modified Rankin scale (95% CI) 3.4 (3.1 to 3.9) 4.4 (4.0 to 4.8) 0.04 Mean absolute infarct growth (interquartile range - mL) 17.7 (-8.8 to 89.2) 60.3 (19.9 to 93.3) 0.10 Outcome and Measure P value Reperfusion # of Patients Partial or complete revascularization # of Patients Figure 14: In IMS III, time from IV to IAT initiation was greater than 2 hours and greatly exceeds IMS I and II. Though likely secondary to decentralization of care secondary to development of PMSC’s, this diminishes the clinical benefit with endovascular therapy in the trial. Figure 15: IMS III patients further suffered a significant lag between groin access and initiation of IAT at the lesion. Fourtyfour minutes is far beyond reported standards with modern guide and distal access catheter technology. IMS III Baseline Characteristics bCTA 70 (23-83) 163 (53.3) 17 (7-40) 177 (57.8) No bCTA 68 (23-89) 177 (50.6) 17 (9-40) 201 (57.4) 123 (33.4) 123.1 (34) Age, median (min-max) Male (%) Baseline NIHSS median (min-max) ASPECTS 8-10 (%) Onset to iv tPA initiation, minutes; mean (SD) IV tPA to Groin Puncture, minutes: mean (SD) 80.7 (26.3)* 90.1 (35.5)* Groin puncture to IA Therapy, minutes: mean (SD) 40.1 (22)** 49.9 (24.2)** Figure 16: Time from puncture to START of IAT was 50 minutes for non cta centers; Centers that did baseline cta's were 20 min faster to IA intervention in IMS III FIGURE 17 RCT for AIS Revascularization: Control arms of PROACT II and NINDS give some indication of LVO natural history Trial Treatment Type Patients NINDS ECASS III IV t-PA vs. Placebo IV t-PA vs. Placebo PROACT II IA pUK + Hep vs. Hep 333 (168 vs. 165) 821 (418 vs. 403) 180 (121 vs. 59) Time Window (hours) 0-3 3-4.5 0-6 Presentation NIHSS 14 vs. 15 10.7 vs. 11.6 17 vs. 17 NR NR 66 vs. 18 mRS < 1 39 vs. 26 mRS < 1 52.4 vs. 45.2 mRS < 2 40 vs. 25 6.4 vs. 0.6 2.4 vs. 0.2 10 vs. 2 Recanalization Outcome (%) at 3 months SICH Rates (%) FIGURE 18 Prospective Intervention Trials: Inferior Outcomes with First Generation Devices Trial IMS- II MERCI Multi- MERCI Penumbra Treatment Type IV t-PA + IA t-PA + EKOS + low dose hep IA Merci + IAT IA Merci + IAT + IVT IA Penumbra + IAT 81 (IAT-55) 141 164 125 Time Window (hours) 0-3 0-8 0-8 0-8 Presentation NIHSS 19 20 19 17 Recanalization (%) 58 60.3 (48 device alone) 68 (55 Merci alone) 81.6 (Device alone) Outcome (%) at 3 months mRS < 2 46 36 36 25 SICH Rates (%) 9.9 7.8 9.8 11.2 Patients FIGURE 19: Outcomes at 3 months better with Reperfusion Study mRS < 2 (%) Mortality (%) MERCI 46 vs. 10.4 (P<0.0001) 31.8 vs. 54.2 (P=0.001) Muti-MERCI 49.1 vs. 9.6 (P<0.001) 24.8 vs. 51.9 (P<0.001) IMS I & II Pooled Analysis 45.6 vs. 6.9 (P=0.004) 10.9 vs. 34.5 (P=0.01)