- Planet 2
advertisement

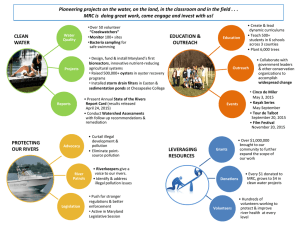
Platelets for Neonatal Transfusion Study 2 (PlaNeT-2): Training Update A randomised controlled trial of platelet transfusion thresholds NHSBT/MRC Clinical Studies Unit NHSBT CTU Neonatal Thrombocytopenia • Prevalence: 1 - 5% of all newborns • 25% NICU admissions • 5-10% severe thrombocytopenia NHSBT Clinical Trials Unit Unit NHSBT/MRC Clinical Studies NHSBT CTU Neonatal Thrombocytopenia: Current Evidence (I) RCT; n= 152 • <1500g; GA < 33weeks; Platelet count – Arm 1: Plt Tx to keep platelet count >150x109/L – Arm 2: Plt Tx at threshold count 50-150x109/L No evidence ‘aggressive’ prophylaxis influenced incidence or extension of IVH Andrew et al (1993) NHSBT Clinical Trials Unit Unit NHSBT/MRC Clinical Studies NHSBT CTU Neonatal Thrombocytopenia: Current Evidence (II) Preterm neonates 4444 Platelet count nadir < 30 17/44 (39%) Given platelet transfusions 15/17 (88%) Not given platelet transfusions 2/17 (12%) Platelet count nadir 30-50 27/44 (61%) Given platelet transfusions 10/27 (37%) Not given platelet transfusions 17/27 (63%) No increased haemorrhage irrespective of whether platelets were administered Murray et al (2002) NHSBT Clinical Trials Unit Unit NHSBT/MRC Clinical Studies NHSBT CTU Neonatal Thrombocytopenia: Current Evidence (III) Retrospective Cohort Analysis of neonates with NEC and platelets <100x109/L • Results suggested platelet transfusions in infants with NEC associated with greater morbidity Kenton et al (2005) NHSBT Clinical Trials Unit Unit NHSBT/MRC Clinical Studies NHSBT CTU Neonatal Thrombocytopenia: Current Evidence (IV) Baer et al (2007) NHSBT Clinical Trials Unit Unit NHSBT/MRC Clinical Studies NHSBT CTU Neonatal Thrombocytopenia: Current Practice Current national transfusion guidance based on consensus rather than evidence British Committee for Standards in Haematology (2004) United Kingdom Blood Services (2007) Survey in the UK showed wide variation in platelet transfusion practice Chaudhary and Clarke (2008) NHSBT/MRC Clinical Studies Unit NHSBT CTU PlaNeT-1: A Study of Outcomes • Prospective observational study of NICU admissions with platelet counts <60x109/L • 7 NICUs • 169 neonates studied for 7 days, or until platelets >60x109/L – Platelet count – Haemorrhage – Platelet transfusions – Outcome Stanworth et al (2009) NHSBT Clinical Trials Unit Unit NHSBT/MRC Clinical Studies NHSBT CTU PlaNeT-1: Haemorrhage NHSBT Clinical Trials Unit Unit NHSBT/MRC Clinical Studies NHSBT CTU PlaNeT-1: Lowest Platelet Counts NHSBT/MRC Clinical Studies Unitgroup median and (- -) IQR NHSBT CTU (++) indicates (++) indicates group median and (- -) IQR PlaNeT-1: Transfusions • 2/3 received platelet transfusion • Most transfusions given as prophylaxis often well after “risk period” for haemorrhage has passed NHSBT/MRC Clinical Studies Unit NHSBT CTU Moving forward! NHSBT Clinical Trials Unit Unit NHSBT/MRC Clinical Studies NHSBT CTU PlaNeT-2 Platelets for Neonatal Transfusion - Study 2 NHSBT Clinical Trials Unit Unit NHSBT/MRC Clinical Studies NHSBT CTU PlaNeT-2: Study design (I) Two-stage, randomised, parallel group, superiority trial. Aim: to compare two different platelet count thresholds for prophylactic platelet transfusion to preterm neonates. Primary Outcome: • Proportion of patients who either die or experience a major bleed up to and including study day 28. NHSBT/MRC Clinical Studies Unit NHSBT CTU PlaNeT-2: Study design (II) Secondary Outcomes: • Proportion of neonates surviving to home following a major bleed • Mortality prior to day 28 • Major bleeds by day 28 • Platelets transfused to study day 28 • Length of hospital stay • Transfusion-related adverse events • Neuro-developmental outcome NHSBT/MRC Clinical Studies Unit NHSBT CTU PlaNeT-2: Platelet thresholds • Arm A Standard: transfuse platelets at <25x109/L (330 neonates) • Arm B Intervention: transfuse platelets at <50x109/L (330 neonates) • Dose: 15 ml/kg NHSBT/MRC Clinical Studies Unit NHSBT CTU PlaNeT-2: Additional platelet transfusions May be considered under the following circumstances: • Therapeutically to treat major bleeding, following objective and documented signs of clinically relevant bleeding graded as moderate, major or severe, but not for minor bleeding. • Prior to planned invasive procedures as below only – Suprapubic aspiration – Lumbar puncture – Major surgery where haemostasis may be critical to outcome. NHSBT/MRC Clinical Studies Unit NHSBT CTU Modified WHO Bleeding Assessment Score Grade 1 – Minor Haemorrhage Any bleed from the skin, umbilical cord, skin around stoma, surgical scar, mucosa. Any pink frothy or old bleed from the ET tube. H1 haemorrhage on cranial US (Germinal Layer Haemorrhage, GLH) Grade 2 – Moderate Haemorrhage Any frank bleed from • the stoma • macroscopic haematuria, • IVH (H2 or H3) without dilatation (V0), • Acute fresh bleed through ETT without ventilatory changes Grade 3 – Major Haemorrhage any: • Frank Rectal • Acute fresh bleed through ETT with ventilatory change. • Intracranial bleed An intracranial bleed is defined as a major bleed if any of the following apply: Neurosurgical intervention is required; Scans show a midline shift; Clinical signs and symptoms of neurolgical deficit with significant derangement of laboratory investigations • Major IVH is defined as H2 or H3 with ventricular dilatation (V1); H1, H2, H3 with parenchymal involvement (P3) ; Any evolution of intracranial haemorrhage to H2V1, H3V1, or (H1, H2, H3) with parenchymal involvement (P3) Grade 4 – Severe Haemorrhage • Shock defined as life threatening major bleed associated with hypotension, hyopovolaemia or any other haemodynamic instability and/or bleeding requiring volume boluses, red cell transfusion in the same 24 hours, fatal major bleeding NHSBT/MRC Clinical Studies Unit NHSBT CTU PlaNeT-2: Inclusion criteria • Admission to a participating NICU (includes postnatal transfers) • <34 weeks GA at birth • Platelet count of <50 x109/L • Cranial ultrasound scan: undertaken <6 hours before randomisation to exclude recent major IVH NHSBT/MRC Clinical Studies Unit NHSBT CTU PlaNeT-2: Exclusion criteria • Major/life-threatening congenital malformations • Recent major haemorrhage within the last 72 hours • All fetal intracranial haemorrhages • Known immune thrombocytopenia • Neonates unlikely to survive • Neonates not given parenteral vitamin K NHSBT/MRC Clinical Studies Unit NHSBT CTU PlaNeT-2: Consent When platelets <100x109/L When platelets <50x109/L Parents will be counselled Documented in the clinical notes Written, informed consent will be obtained Three copies signed: •For parents •For the clinical notes with Parent Information Sheet •For the study file NHSBT/MRC Clinical Studies Unit NHSBT CTU Parents will be approached for consideration of immediate study participation. Document on PlaNeT-2 log book PlaNeT-2: Randomisation When the consent is signed and platelets <50x109/L: Prerandomisation Platelet transfusion information (FA) Prerandomisation form (F1) NHSBT/MRC Clinical Studies Unit NHSBT CTU Eligibility for randomisation (F2) Current medical conditions & previous major bleeds (F3) Randomisation (F4) FA – Platelet transfusion information NHSBT/MRC Clinical Studies Unit NHSBT CTU F1 – Pre-randomisation NHSBT/MRC Clinical Studies Unit NHSBT CTU F2 – Eligibility for randomisation NHSBT/MRC Clinical Studies Unit NHSBT CTU F3 – Current medical conditions And previous major bleeds NHSBT/MRC Clinical Studies Unit NHSBT CTU F4 – Randomisation NHSBT/MRC Clinical Studies Unit NHSBT CTU PlaNeT-2: Data collection (I) SD1 SD2 SD3 SD4 SD5 SD6 SD7 SD8 SD9 SD10 SD11 SD12 SD13 SD14 BAT BAT BAT BAT BAT BAT BAT BAT BAT BAT BAT BAT BAT BAT USS USS SD15 SD16 SD17 SD18 SD19 SD20 SD21 SD22 SD23 SD24 SD25 SD26 SD27 SD28 BAT BAT BAT BAT BAT BAT BAT BAT BAT BAT BAT BAT BAT BAT USS SD29 SD30 SD31 SD32 SD33 SD34 SD35 Weekly NHSBT/MRC Clinical Studies Unit NHSBT CTU USS SD36 SD37 … … … … END USS F5 – Bleeding Assessment Tool (BAT) NHSBT/MRC Clinical Studies Unit NHSBT CTU F7 – Weekly Data Collection NHSBT/MRC Clinical Studies Unit NHSBT CTU PlaNeT-2: Data collection (II) F8 Platelet Transfusion Data (F8) F9 F10 Discontinuation of Treatment Allocation (F10) F13 Major/Severe Bleed (F13) F14 F15 Necrotising enterocolitis (NEC) /Sepsis Form (F9) Serious Adverse Event (F14) Serious Platelet Transfusion Related Adverse Event (F15) NHSBT/MRC Clinical Studies Unit NHSBT CTU F8 – Platelet Transfusion Data NHSBT/MRC Clinical Studies Unit NHSBT CTU PlaNeT-2: NEC/Sepsis form • Necrotising enterocolitis ≥ Stage 2 defined as per Bells Criteria (Bell et al,1978) • Sepsis: culture positive sepsis or culture negative sepsis where a course of at least 5 days of antibiotics is to be administered for proven or clinically-suspected sepsis. • All episodes of NEC and sepsis must be recorded on the adverse event form • A listing of adverse events will be reported six monthly to the Independent Data Monitoring Committee. NHSBT/MRC Clinical Studies Unit NHSBT CTU F9 – NEC/Sepsis form NHSBT/MRC Clinical Studies Unit NHSBT CTU F10 – Discontinuation of Treatment Allocation NHSBT/MRC Clinical Studies Unit NHSBT CTU PlaNeT-2: Major/Severe bleed form • All new major bleeding events will be reported to the CSU without disclosing allocation arm. • Each report will be forwarded to the Independent Data Monitoring Committee for review as soon as it is received at the CSU. • In cases of uncertainty the local team may contact one of the CIs or neonatal medical experts. MAJOR BLEED FORM Within one working day of becoming aware of an Major Bleed, please NHSBT/MRC Clinical Studies Unit fax a completed Major Bleed form to the NHSBT/MRC CSU Fax: 01223 588136 F13 – Major/Severe Bleed NHSBT/MRC Clinical Studies Unit NHSBT CTU PlaNeT-2: Serious Adverse Event (SAE) A SAE is an adverse event that results: • • • • in death is life-threatening requires hospitalisation or prolongation of existing hospitalisation (including readmission within 28 study days if discharged home earlier) there is a likelihood of persistent or significant disability or incapacity SAE NOTIFICATION Within one working day of becoming aware of an SAE, please fax a completed SAE form to the NHSBT/MRC Clinical Studies Unit NHSBT/MRC CSU Fax: 01223 588136 F14 – Serious Adverse Event NHSBT/MRC Clinical Studies Unit NHSBT CTU PlaNeT-2: Serious platelet transfusion related adverse event Data collected on serious transfusion related adverse reactions/events will be based on current definitions used by hospitals reporting to UK national haemovigilance reporting schemes (SHOT and MHRA). These definitions cover the following: Incorrect blood component transfused Acute transfusion reactions Transfusion Related Acute Lung Injury (TRALI) Transfusion transmitted infections, including bacterial transmission Transfusion Associated Circulatory Overload (TACO) NHSBT/MRC Clinical Studies Unit NHSBT CTU F15 – Serious platelet transfusion related adverse event NHSBT/MRC Clinical Studies Unit NHSBT CTU PlaNeT-2: End of study Data collection will cease when the baby is 38 weeks corrected gestational age or time of discharge home Cranial Ultrasound at End of Study (F11) End of Study (F12) NHSBT/MRC Clinical Studies Unit NHSBT CTU F11 – Cranial USS at the end of study NHSBT/MRC Clinical Studies Unit NHSBT CTU F12 – End of study NHSBT/MRC Clinical Studies Unit NHSBT CTU PlaNeT-2: Transfer out of recruiting unit • Inform the local PlaNeT-2 team if neonate transferred out of recruiting unit • Keep all the forms, do not send them with the patient • Receiving hospital should: • Collect information as required by the protocol thereafter until 38 weeks CGA or discharge. • Continue to give any required platelet transfusions according to the randomised platelet threshold. NHSBT/MRC Clinical Studies Unit NHSBT CTU PlaNeT-2: Data Quality • Black ink • For platelet count use the date and time the sample is received in the lab • Do not leave blank fields, enter leading zeros • Any data incorrectly recorded must be crossed through with a single line and the correct value documented by the side. All corrections must be initialled and dated by the individual making the changes NHSBT/MRC Clinical Studies Unit NHSBT CTU PlaNeT-2: Research & Development and Ethics approval • Ethics approval obtained from the regional ethics committee • NIHR adopted • CLRN adopted NHSBT/MRC Clinical Studies Unit NHSBT CTU PlaNeT-2: Trial Governance The Trial Management Group (TMG) will be responsible for the daily management of the trial. The TMG is responsible to the Trial Steering Committee which has oversight of the trial and provides advice as needed NHSBT/MRC Clinical Studies Unit NHSBT CTU PlaNeT-2: TMG Group • • • • • • Louise Choo Paul Clarke Anna Curley Alison Deary Rizwan Khan Renate Hodge NHSBT/MRC Clinical Studies Unit NHSBT CTU • • • • • • Priya Muthukumar Helen New Simon Stanworth Vidheya Venkatesh Tim Watts Karen Willoughby PlaNeT-2: Finance The trial is funded by a Project Grant from the NHSBT which is administered by the National Research & Development Committee. Any payments will be made to participating centres to cover costs associated with the undertaking of this trial, as specified in Individual Investigator Site Agreements. NHSBT/MRC Clinical Studies Unit NHSBT CTU PlaNeT-2: Communications • For general trial queries: contact • Karen Willoughby kw369@medschl.cam.ac.uk, copy to Alison.Deary.alison.deary@nhsbt.nhs.uk • For medical queries regarding transfusions, SAEs, grading major/severe bleeds or transfusion reactions contact • Simon Stanworth: simon.stanworth@nhsbt.nhs.uk, or • Anna Curley: anna.curley@addenbrookes.nhs.uk www.planet-2.com NHSBT/MRC Clinical Studies Unit NHSBT CTU References • Andrew M, Vegh P, Caco C, Kirpalani H, Jeffries A, Ohlsson A, Watts J, Sagial S, Milner R, Wang EA. (1993) Randomised controlled trial of platelet transfusions in thrombocytopenic premature infants. Journal of Pediatrics 123 285–291. • Baer VL, Lambert DK, Henry E, Snow GL, Sola-Visner MC, Christensen RD (2007) Do platelet transfusions in the NICU adversely affect survival? Analysis of 1600 thrombocytopenic neonates in a multihospital healthcare systemMultiple platelet transfusions and survival. Journal of Perinatology 27 790-796. • British Committee for Standards in Haematology (2004) Transfusion Guidelines for neonates and older children. British Journal of Haematology 124 433-453. • Chaudhary R, Clarke P. (2008) Current transfusion practices for platelets and fresh, frozen plasma in UK tertiary level neonatal units. Acta Pædiatrica 97(1) 135. • Kenton AB, Hegemier S, Smith EO, O'Donovan DJ, Brandt ML, Cass DL, Helmrath MA, Washburn K, Weihe EK, Fernandes CJ (2005) Platelet transfusions in infants with necrotizing enterocolitis do not lower mortality but may increase morbidity. Journay of Perinatology 25(3) 173–177 • Murray NA, Howarth LJ, McCloy MP, Letsky EA, Roberts IAG (2002) Platelet transfusion in the management of severe thrombocytopenia in neonatal intensive care unit patients. Transfusion Medicine 12(1) 35-41. • Stanworth S, Clarke P, Watts T, Ballard S, Choo L, Morris T, Murphy MF, Roberts I (2009) Prospective, observational study of outcomes in neonates with severe thrombocytopenia. Pediatrics 124 826-834. • United Kingdom Blood Services (2007) Handbook of Transfusion Medicine. 4th ed. Norwich: The Stationary Office. [online]. Available from: http://www.transfusionguidelines.org.uk/index.aspx?Publication=HTM NHSBT/MRC Clinical Studies Unit NHSBT CTU Thank you! NHSBT/MRC Clinical Studies Unit NHSBT CTU