CD8 T
advertisement

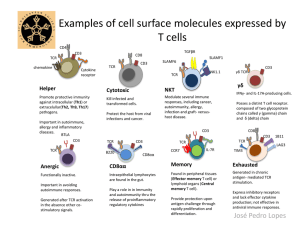
Treg exert differential effects on the proliferation and differentiation of CD8 T cell subsets in chronic HIV-1 infection M. Nikolova1, M. Muhtarova1, M. Younas2, J.D. Lelievre2,3, H. Taskov1, Y. Levy2,3 1National Center of Infectious and Parasitic Diseases, Sofia, Bulgaria Paris Est Créteil, Inserm U955, Créteil, France 3Henri Mondor Hospital, APHP, Créteil, France 2Université XVIII International AIDS Conference | July 18-23 2010 | Vienna, Austria CD8 T cell differentiation and functional maturation Differentiation IFNg Cytotoxicity Proliferation Naïve Central memory CD45RA+ CCR7+ CD28+ CD27+ Effector Memory Effector memory 1 Effector memory 2 CD45RA-/CD45RO+ Effector Terminal effector CD45RA+ Background & Rationale CD8 T memory/effector subset balance determines the control of chronic viral infections HIV infection is characterized by a decreased proliferative capacity of CM CD8 T cells and incomplete differentiation of HIV-specific effector T cells (Appay V. et al, J. Exp. Med 2000; Champagne P. et al, Nature 2001) We have previously shown that Treg (CD4+CD25highFoxP3+) influence M/E CD8 subset balance in HIV- donors: Treg inhibit the proliferation of effectors and the differentiation of memory CD8 T upon polyclonal and antigen-specific in vitro stimulation (Nikolova M. et al, Blood, 2009) Treg are expanded in acute and chronic HIV-1 infection, and inhibit effector CD8 T cell responses in vitro (Weiss L. et al, Blood 2004; Kinter A. et al, J. Exp. Med 2004) HYPOTHESIS AND OBJECTIVE We hypothesized that the expansion of Treg in HIVinfected patients might contribute to the dysbalance between effector and memory CD8 T cells Our objective was to investigate the effects of Treg on the proliferation and maturation of different CD8 T cell subsets in chronic HIV-1 infection STUDY POPULATION HIV-1+cART+ subjects (n=14), CD4 >350 cells/ml, VL < 50 HIV RNA copies/ml STUDY DESIGN PMNC D0, flow cytometry (CD45RA/CCR7/CD27/CD28/CD3/CD8) Treg depletion, anti-CD25 DynaBeads proportions of CD8 T subsets PMNC,Treg- PMNC, Treg+ CFSE staining, polyclonal stimulation: immobilized anti-CD3 (5 mg/ml) D5, flow cytometry (CFSE/CD45RA/CCR7/CD27/CD28/CD3/CD8) COMPARISON proportions and proliferation rates of CD8 T subsets CD8 T SUBSETS PHENOTYPING : GATING STRATEGY CD8 T CELL SUBSETS: PROLIFERATION RATES Gated CD3+CD8+ Ly Gated CD8 T subset Treg+ Treg- N E M CFSElow 81% CFSElow 68 % CD45RA TE CD27 1. CD27+CD45RA+ Naïve, N 2. CD27+CD45RA- Memory, M 3. CD27- CD45RA- Effector, E 4. CD27- CD45RA+ Terminal Effector, TE CFSE CFSE-stained Treg+ and Treg- PMNC were stimulated with 5mg/ml immobilised anti-CD3 % CFSElow cells was determined on D5 Polyclonal stimulation in the presence of Treg results in a decreased rate of CD8 T cell proliferation ** 100 % CFSElow CD8 T 90 80 70 60 50 40 Treg+ Treg+ CD8 TregTregCD8 Proliferation rates of Treg+ and Treg- CD8 T (n=14, D5 anti-CD3), mean 72 vs. 81 % CFSElow CD8 T ** p<0.01, Student’s T-test Polyclonal stimulation in the presence of Treg results in lower proliferation rates within E and TE subsets , while M CD8 are not significantly affected * ** * 100 80 60 Treg+ Treg- 40 20 0 N CD27+45RA+ CD27+45RAM CD27-45RAE CD27-45RA+ TE %CFSE low CD8 T %CFSE low CD8 T 100 ** 50 0 E TE * p<0.05, **p<0.01, Student’s T-test Proliferation rates of Treg+ and Treg- CD8 T subsets (D5, anti-CD3); av. 46% vs. 74% E, (P< 0.05) and 48% vs. 85% TE, (P< 0.01) have proliferated Treg inhibit the differentiation of polyclonally stimulated naive CD8 T cells into CD27-CD45RA- effectors 60 % of CD8 T cells ns 50 * 40 * 30 ns 20 D0 D5,Treg+ D5,Treg- 10 0 CD27+RA+ N CD27+RA- M Cd27-RA- E CD27-RA+ TE Distribution of CD8 T subsets before and after polyclonal stimulation in the absence or in the presence of Treg; 19 and 27 % E CD8 were observed in the presence and in the absence of Treg, respectively (* p<0.05, n=14, Student’s T-test) Analysis of memory CD8 T cells: CM/EM Gated CD3+CD8+ Ly Gated CD45RA-CD27+CD8 T EM1 CD45RA CD28 CM M CD27 EM2 CM CCR7 Analysis of CD28/CCR7 co-expression on M (CD27+CD45RA-) CD8 T cells after polyclonal stimulation (D5, anti-CD3) in the absence or in the presence of Treg Polyclonal stimulation in the presence of Treg results in a significant increase of CM (CCR7+) CD8 T cells 70 % of M CD8 T cells 60 50 40 NA ** * D5,Treg+ 30 D5, Treg- 20 10 0 CM CCR7+ EM1 R7-28+ EM2 R7-28- Composition of the M CD8 T cell subset before and after polyclonal stimulation, in the absence or in the presence of Treg. (n=14, **p<0.05, **p<0.05 in comp. to D0, Student’s T-test) Polyclonal stimulation in the presence of Treg results in a significant increase of CM (CCR7+) CD8 T cells ** % of M CD8 T cells 60 * 50 D0 40 Treg+ 30 Treg- 20 10 0 NS NS Treg+ Treg- D5, anti-CD3 Proportions of CM cells within the memory (CD27-CD45RA+) CD8 T cell subset before and after 5 days anti-CD3 stimulation in the presence or in the absence of Treg: Average % of CM CD8 were 15.5 vs. 24 and 16 respectively. (n=14, *p<0.05, **p<0.01, Student’s T-test) HIV+ specific CD8 T are mostly of EM2 phenotype CD8 T cell differentiation Treg IFNg Cytotoxicity Proliferation Naïve Central memory CD45RA+ CCR7+ CD28+ CD27+ Effector Memory Effector memory 1 Effector memory 2 CD45RA-/CD45RO+ Effector Terminal effector CD45RA+ Conclusions Increased frequency of Treg in HIV infection significantly decreases the rate of CD8 T cell proliferation, affecting specifically E and TE subsets. Polyclonal stimulation of CD8 T cells in the presence of Treg results in decreased differentiation of effectors and in accumulation of CM cells. Globally, these results indicate that the expansion of Treg in the settings of HIV infection and generalized immune activation may contribute to the dysbalance between M and E antigen-specific CD8 T cells The questions to answer Subset-specific effects of Treg at the level of HIV-specific CD8 T cells Subset-specific effects of Treg at the level of other virus-specific CD8 T cells Mechanisms of Treg subset-specific effects in HIV infection… PD1/PDL1? ACKNOWLEDGEMENTS Henri Mondor Hospital, APHP, Université Paris Est Créteil, Inserm U955, Créteil, France Dr. Matthieu Carrière Dr. Mohammad-Ali Jenabian Dr. Christine Lacabaratz Pr. Jean-Daniel Lelièvre Pr. Yves Lévy PHC Rila 2009 (Bulgarian Ministry of Education and Sience / Ministry of Foreign and European Affairs, France; Sidaction - France; ELTA’90 Ltd - Bulgaria National Reference Laboratory of Immunology, National Center of Infectious and Parasitic Diseases, Sofia, Bulgaria Dr. Maria Muhtarova Dr. Draganka Stankulova Antoaneta Mihova Pr. Hristo Taskov