PPT Presentation
advertisement

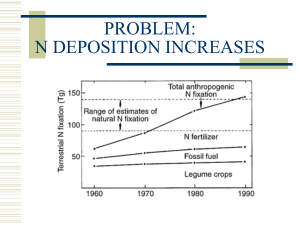
15N in marine plants Modified by Angela Quiros There is lots of variation in the 15N values in the world’s oceans. (Montoya 2007) Outline • Broad processes & Inputs • How much nitrate is used up • Case Study: Seasonal processes in the Eastern North Pacific • Nitrogen isotopes in seagrass Major Inputs of Nitrogen in the Ocean 1. Deep Water: waters ~4.5 ‰ • Upwelled nitrate 2. Atmospheric deposition: waters ~0 ‰ • Largest in areas near continental land masses 3. Nearshore and continental shelf waters • Terrigenous runoff may be a large source • Heavy if fecal material, light if agricultural input, soil signature if relatively pristine 4. N-fixation from the atmosphere: waters ~0 ‰ Major Processes • N2 fixation • Nitrification • Denitrification Major Processes • N2 fixation: 15N ~0‰ for phytoplankton; waters ~0 ‰ - Inert N2 from the atmosphere converted to ammonia NH3. Diazotrophs fix N, symbiotic w/ diatoms occur in dense blooms, impt part of phytoplankton, contribute a lot to local N budget N2 + 6H+ = 6e -> 2NH3 -> NH4+ (ammonium cation) - Produces organic matter depleted in 15N relative to deepwater NO3-, so it lowers the 15N, while adding to the pool of combined N - BUT…low value could also be an indication of recycled NH4+ being used in oligotrophic waters - In Bermuda, 15NO3- (nitrate) is 2.8‰ lower than oceanic average because of N2 fixation. The importance of N-fixation in oligotrophic waters: Trichodesmium abundance and d15N of zooplankton • d15N values lowest with highest abundance of Trichodesmium, • -1 to -2 ‰ • d15N values highest in areas with low abundance of Trichodesmium • waters ~0 ‰ Major Processes • Nitrification: N available through upwelling & convection • Biological oxidation of ammonia w/ oxygen into nitrite then nitrate, significant isotopic fractionation, a source of depleted N in water column. • Mineralization is the complete decomposition of organic material, release of available N, replenishing N cycle NH3 + O2 -> NO2 + H2O -> NO3- 15N depends on regional processes - 15N >0 - NH4+ : available from urea - typically lighter than the global ocean average; 15N is low Major Processes • Denitrification – waters isotopically heavy: waters ~8 ‰ • Microbially facilitated, reduce nitrate to produce N2 NO3- -> NO2 -> NO -> N20 -> N2 (gas) • Shows fractionation, lighter isotopes of N preferred, leaving heavier N istopes in residual matter • Discrimates strongly against 15N; negative delta values -40 ‰ • In oxygen minimum zones, denitrifying bacteria use NO3- as an electron acceptor to support heterotrophic growth, reducing it to N2. • In major pelagic oxygen minimum zones, denitrification consumes only a part of available NO3-, so there is a significant enrichment of residual NO3- (15-18 ‰) Outline • Broad processes & Inputs • How much nitrate is used up • Case Study: Seasonal processes in the Eastern North Pacific • Nitrogen isotopes in seagrass Where in the world is the Nitrogen? Natural abundance of N stable isotopes vary with marine ecosystem Nitrate QuickTime™ and a decompressor are needed to see this picture. All marine autotrophs besides N2-fixing prokaryotes need combined N: nitrate (NO3-), nitrite (NO2-), ammonium (NH4+), typically 4-5‰. (Montoya 2007) Global Average d15NO3- ~4‰ - 5‰ global 15N values of deep water Deepwater NO3- is the largest pool of combined N in the ocean. N2-fixation adds to it, while denitrification removes N from it Different areas of the world are on different parts of this curve… QuickTime™ and a decompressor are needed to see this picture. If N in = N out, then product is lighter than the initial N, but as the pool of N is used, the product (phytoplankton) gets heaver. If all the N is used, the product (phytoplankt) N = nitrate value. (Montoya 2007) QuickTime™ and a decompressor are needed to see this picture. Particulate Organic Nitrogen (Montoya 2007) Nitrogen in the Ocean • a. b. c. PON- Particulate Organic Nitrogen Rapidly sinking particles (marine snow) Slowly sinking particles Upwelled PON from below the euphotic zone (Michener & Kaufman 2007) PON plays a role in vertical transport of material out of the euphotic zone • • • 15N of PON will determine the 15N of phytoplankton Zooplankton are ammonotelic, so deamination rxns produce NH4+ depleted in 15N, there is a preferential loss of 14NH + & an enrichment of 4 15 the N in the body. 14N is retained in the upper water column through tight recycling. Rapidly sinking particles transport 15N into the deep ocean QuickTime™ and a decompressor are needed to see this picture. Using isotopes to trace a phytoplankton bloom…isotopic transients QuickTime™ and a decompressor are needed to see this picture. • Phytoplankton fractionate 15N during assimilation of nitrate, so preferential uptake of 14NO3 by phytoplankton. At the start of a bloom, production of organic matter is depleted in 15N, relative to available NO3-. As bloom progresses, preferential removal of 14NO3- increases the 15N of residual NO3- pool. • Zooplankton lag behind (Montoya 2007) Outline • Broad processes & Inputs • How much nitrate is used up • Case Study: Seasonal processes in the Eastern North Pacific • Nitrogen isotopes in seagrass Upwelling equatorward winds surface waters nutrient rich water from depth The nitrogen isotope biogeochemistry of sinking particles from the margin of the Eastern N. Pacific (Altabet et al 1999) -Collected sediment traps, water column samples -Isotopic analysis of NO3from seawater -Compared time series sediment traps w/ material QuickTime™ and a decompressor fluxes & compared are needed to see this picture. sediment traps with actual sediments N isotopes in sinking particles in the Eastern N. Pacific • Upwelling- filament of cold, nutrient rich water brought to the surface from 1 section of the coast, advection at the surface; direction south or offshore • Episodes of high productivity & particle flux • El Nino results in sharp reduction of nutrients because no persistent upwelling & nutrient rich water is deeper • CA Undercurrent- coast to 100km offshore, 10/20m-600m, core @ 150m, source of upwelling Nitrogen Fixation • Lower than average d15N values • d15N of sediment increases with depth, so it’s hard to use sediments to map phytoplankton! • Isotopically light sinking organic matter lowers the d15N of the subsurface pool below the global deep water average • Subsurface pool d15N is a mix between particle flux from the surface and vertical mixing of deep water (Altabet et al 1999) Nutrient Profiles – Monterey Bay • Inverse relationship between [NO3-] and d15NO3- (nitrate) • d15NO3- decreases with depth due to remineralization of sinking particles • d15NO3- mean is 8‰, which is higher than oceanic values (4‰ 5‰) – probably due to infusion of California undercurrent waters and denitrification (Altabet et al 1999) Nutrient Profiles – Gulf of California • Surface waters are enriched compared to Monterey profile • Increase in d15N at the surface is most likely due to uptake by phytoplankton •Nitrate drawdown (by denitrifying bacteria) within OMZ corresponds with increase in d15N, mean 10-12‰ because there is more denitrification in the south. Denitrification makes N heavy. (Altabet et al 1999) Particles get heavier as you go deeper QuickTime™ and a decompressor are needed to see this picture. But time-series data show lots of variation! (Altabet et al 1999) QuickTime™ and a decompressor are needed to see this picture. N isotopes in sinking particles in the Eastern N. Pacific • New N or other NO3- not significant contributors • Sediment traps are good paleoceanographic records for 15NO3• Denitrification is the principle modifier for subsurface NO3- responsible for >8‰ vs open ocean is 4.6‰ • 15NO3- high in Monterey & San Pedro even though they are not zones of active water column denitrification because the ETNP supplies 15NO3to subsurface waters Bottom Line… d15N of phytoplankton depends on: • denitrification • nitrogen fixation • upwelling and currents Outline • Broad processes & Inputs • How much nitrate is used up • Case Study: Seasonal processes in the Eastern North Pacific • Nitrogen isotopes in seagrass QuickTime™ and a decompressor are needed to see this picture. Food web integrators & environmental tracers Nitrogen isotopes in seagrass • 15N of seagrass (S.G) varies from -2‰ to 12.3‰, with most frequent values 0 to 8‰ • Variations in isotopic ratio are due to inorganic N incorporation from the water column and sediment • 15N close to 0‰ are due to N2 fixation by associated S.G. organisms Nitrogen Isotopes in Seagrass • Food webs • • • 15N used to assess food webs because of the 15N enrichment with increasing trophic position Environment Isotopic signatures from nitrate in wastewater, fertiliser, atmospheric deposition. Wastewater has higher 15N because of human sources & isotopic discrimination during remineralisation • Used to map sewage because longer turnover time