CO 2
advertisement

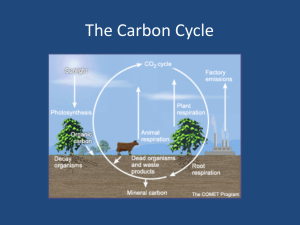
Global Carbon Cycle Why study the C cycle? • Key element of life – so fundamental – Fossil fuel burning and global warming • Perturbation by humans (atm CO2) • Complex cycle – long & short term cycles; organic and inorganic components • Geological processes operating over millions of years • Biological processes operating on annual time scales • Interactions between long and short term cycles “short”-term “long”-term anthropogenic Combined Inventories Atmosphere 770 Gt C Terr. Systems ~2400 Gt C Oceans ~39,000 Gt C Sed. Rocks 50,000,000 Gt C 1. 2. 3. 4. Fig. 8-3 Major Inventories Majority of C tied up in rock cycles – large reservoirs with long residence times Reservoirs active on short time scales are ocean, atm, & land Large exchange fluxes to and from atm – atm has short residence time (3 yr); small net fluxes due to biology (most PP is respired) Problem with adding fossil fuel CO2 to atm – transferring C from long term geologic reservoir to a short term reservoir – may affect short term feedback control mechanisms 50.3 Atmospheric CO2 Respiration Air-Sea Exchange Terrestrial primary production and respiration Physical weathering 59.6 - 59.7 50 CO2 POC Marine primary prod. River transport 60 0.4 Upwelling remineralization Kerogen Land Plants Particle Rain Oceans Humification Uplift of sedimentary rocks POC Deposition CO2 Benthic Fluxes Soil humus 0.1 Recent Sediments 0.1 Carbon Burial Sedimentary Rocks A model – little transfer of biological C to ocean (from land) or sediments (from water) Atmosphere • Most C in atm as CO2 – Some methane and CO • Atm CO2 shows rapid increase in recent time – Beginning with Industrial Revolution • See seasonal variations in recent increase – Uptake in Spring due to plant growth (N hemisphere) – Release in fall from net respiration Northern hemisphere -More land -More terrestrial prod Figs. 1-2 and 1-3 Southern amplitude is lower Seasonality offset by 6 mos. 400 South Pole Barrow, AK CO2 (p p m ) 380 360 340 320 300 1975 1980 1985 1990 1995 2000 Year Northern hemisphere has more extensive seasonal forests Close tracking between N & S hemispheres 2005 Prior to humans, the system showed natural variability (50 – 80 ppm glacial-interglacial) Smaller Holocene changes Interglacial Glacial Holocene changes • Recent high resolution ice core • Natural variability in Holocene is second order change when compared with glacialinterglacial excursions and anthropogenic increase • Allows us to think about a nearly constant pre-industrial interglacial CO2 level of ~280 ppm Recent increases in Atm CO2 • Some due to land use changes (preindustrial) – Deforestation – two-fold problem • Decrease PS uptake of CO2 • Burn the wood - charcoaling • Mainly due to fossil fuel burning (postindustrial) • Deforestation in tropics may be partially balanced by N hemisphere forest expansion/regrowth Atmos. increase (3.3 PgC/yr) IPCC – Intergovernmental Panel on Climate Change Land use change (1.7 PgC/yr) Emissions (5.5 PgC/yr) Atmosphere Residual terrestrial sink (1.9 PgC/yr) Ocean uptake (2 PgC/yr) Atmosphere CO2 ∆pCO2 > 0 (primarily upwelling regions) ∆pCO2 < 0 (primarily high latitudes) CO2 CO2 Surface Ocean CO2 + CO32- + H2O 2HCO3- CO2 + H2O Organic Matter + O2 Ca2+ + 2HCO32- CaCO3 + CO2 + H2O CO2 + CO32- + H2O 2HCO3- Upwelling and vertical mixing Sinking particulate organic matter (“biological pump”) CO2 + H2O Organic Matter + O2 Deep Ocean HCO3- Bottom water formation (high latitudes) (“solubility pump”) CO2 CO2 + H2O Organic Matter + O2 Ca2+ + 2HCO32- CaCO3 + CO2 + H2O Sediments Oceans are largest “active” reservoir in the carbon cycle – primarily DIC Oceans • Link the “active” or short-term cycles with long-term geological cycles – sink for fossil fuel CO2 • Ocean processes – Biological cycle – Weathering reactions and long term controls – Atm CO2 riverine bicarb neutralized in ocean returned to atm or buried in seds • Processes that remove CO2 from atm – Gas exchange – equilibration of sfc ocean with atm – Biological pump – Bottom water formation Gas exchange • If CO2 were a simple gas, ocean could only take up ~3% of fossil fuel input • Acid-base chemistry enhances ocean uptake • Remember carbonate buffering system? – CO32- + H2O + CO2 2 HCO3– Buffering rxn drives CO2 to bicarb • Surface waters reach equilibrium with atm in about 1 year – Can keep pace with human activity – But surface ocean too small to have capacity to remove it all Biological Pump • PP and calcite ppt consume DIC • Removed from surface ocean via particle flux • Through interactions with carbonate system, this lowers partial pressure (pCO2) in surface ocean which enhances gas exchange (DpCO2< 0) • Transports CO2 to deep ocean in the form of OM or calcite shells • Limitations of biological pump – Availability of other nutrients (N, P, Fe) – More CO2 doesn’t necessarily lead to more PP Bottom water formation • Removes CO2 by physical movement of water away from surface • Solubility pump • CO2 is more soluble in cold water Intermediate and deep water • Can add CO2 through oxidation of OM • Calcite dissolution – excess CO2 from OM oxidation reacts with sinking calcite Upwelling • Intermediate waters are enriched in DIC – Mixing with deep waters, OM oxidation & calcite dissolution, yields some CO2 increase • Upwelling results in excess pCO2 in surface waters (DpCO2 > 0) – Oceans outgas CO2 – High productivity upwelling can still be net CO2 sinks Global oceanic C sources and sinks for atm C - reflect upwelling and deep water formation and high productivity IPCC calculations • Integrate data on ocean flux data • Calculation attempts to assess short-term sinks for excess atm CO2 due to anthropogenic activities Time scales of ocean C cyle • Ocean processes slow relative to rate of fossil fuel burning • Bottom water circulation on timescales of 100’s of years so equilibration with atm is slow • Deep sea seds equilibrate with atm on timescales of 1000’s of years – where the bulk of the ocean’s neutralizing capacity resides • Oceans respond too slowly to take up all excess CO2 – so atm CO2 is increasing • But, oceans have helped! Oceans have taken up 1/3 to ½ of added CO2 Atmosphere ∆pCO2 > 0 (North Pacific and upwelling regions) CO2 CO2 ∆pCO2 < 0 (primarily high latitudes) Equilibration time ~1 yr CO2 Surface Ocean CO2 + H2O Organic Matter + O2 CO2 + CO32- + H2O 2HCO3- Ca2+ + 2HCO32- CaCO3 + CO2 + H2O CO2 + CO32- + H2O 2HCO3- Upwelling and vertical mixing Deep Ocean Sinking particulate organic matter (“biological pump”) Equilibration time ~500-1000 yr CO2 + H2O Organic Matter + O2 HCO3- Bottom water formation (high latitudes) CO2 CO2 + H2O Organic Matter + O2 Ca2+ + 2HCO32- CaCO3 + CO2 + H2O Sediments Equilibration time ~103-104 yr Terrestrial systems • Variety of reservoirs that turnover on different timescales – Soil humus – altered remains of plants – Land plant biomass – Methane – source of atm methane • Terrestrial PP ~ = to Marine PP • Terrestrial systems store excess CO2 differently – humus versus bicarb • Imp for understanding system responses to increasing CO2. Increasing CO2: – might increase PP (neg feedback) – might increase rates of decomposition (pos feedback) 50.3 Atmospheric CO2 Respiration Air-Sea Exchange Terrestrial primary production and respiration 59.6 - 59.7 Physical weathering 50 CO2 POC Marine primary prod. River transport 60 Upwelling 0.4 remineralization Kerogen Land Plants Particle Rain Oceans Humification Uplift of sedimentary rocks POC Deposition CO2 Benthic Fluxes Soil humus 0.1 Recent Sediments 0.1 Carbon Burial Sedimentary Rocks Comparable terrestrial & marine PP negative feedback (temperature and CO2 fertilization) Terrestrial system responses to rising CO2 and global warming positive feedback (temperature enhancement of soil respiration) Controls on atm CO2 • Break down overall cycle to components • Look at effects on particular components Atmospheric CO 50.3 Air-Sea Exchange Respiration Terrestrial primary production and respiration 50 CO 2 POC Marine primary prod. 59.6 - 59.7 Physical weathering 2 60 River transport Upwelling 0.4 remineralization Kerogen Land Plants Particle Rain Oceans Humification Uplift of sedimentary rocks POC Deposition CO 2 Benthic Fluxes Soil humus 0.1 Recent Sediments 0.1 Sedimentary Rocks Carbon Burial Short-term biological cycle • • • • • Years to decades Does not include calcite ppt/dissolution Does not include anthropogenic inputs PS versus respiration nearly balanced – little loss Some transport of org C from land to oceans – Most gets oxidized in the ocean • Small amount of marine OM buried in seds – Leaves behind some O2 in atm • Short-term cycles process a lot of CO2 - 30-50% of atm CO2 consumed per year O2 Net productivity Q uickTim e™ and a TI FF ( LZW) decom pr essor ar e needed t o see t his pict ur e. CO2 Organic matter O2 Net productivity Q uickTim e™ and a TI FF ( LZW) decom pr essor ar e needed t o see t his pict ur e. CO2 Organic matter O2 Net productivity CO2 Uplift and kerogen oxidation O2 Q uickTim e™ and a TI FF ( LZW) decom pr essor ar e needed t o see t his pict ur e. CO2 Organic matter CO 2 H 2 O CH 2 O O 2 O2 Net productivity CO2 Uplift and kerogen oxidation O2 Q uickTim e™ and a TI FF ( LZW) decom pr essor ar e needed t o see t his pict ur e. CO2 Organic matter Long term org C cycles • Millions of years • Components include: OM in sediments, fossil fuels, atm O2 versus CO2 • Burial of OM from Short-term cycle – Inc P and T; most ends up as kerogen, – Some winds up in fossil fuels (oil, coal) – OM in shale is largest reservoir on earth (long t) • Removal balanced by kerogen oxidation/weathering • Affects atm O2 – Net burial leaves O2 in the atm Also linked to pyrite burial/oxidation which requires OM as an intermediate to catalyze the sulfate reduction CO 2 H 2 O CH 2 O O 2 2 Fe 2 O 3 16 Ca 2 16 HCO 3 2 8 SO 4 4 FeS 2 16 CaCO 3 8 H 2 O 15 O 2 Produced by bacterial sulfate reduction - linked to carbon oxidation O2 in atm controlled by a balance between pyrite and OM burial in seds and later oxidation on land Without this balance atm O2 would increase to 150% of present Levels and depletion of atm CO2 in < 10,000 years (see text) Long-term inorg C cycle • 100’s of millions of years • Balance between weathering and plate tectonics – Weathering silicate rocks consumes CO2, transferred to the ocean as bicarb, removal of bicarb by organisms & calcite, burial in seds, subduction, vulcanism (also affects other cations – Ca, Mg, Na) • Cycle is a balance between weathering (takes up CO2) and tectonics (releases CO2) • Plate tectonics – more vigorous then more CO2 release • Climate sensitivity (weathering) CaCO3 ppt. Bicarbonate transport CO2 removal CaCO3 (“regenerates” CO2) Figs. 8-17 (“regenerates” CO2) Short-term Long term (organic Long term (inorganic/tectoni Link with short term C cycle In surface oceans “adds” back CO2 Onset of modern plate tectonics “turns this on” Increase in surface temperature due to increase in solar luminosity Drop in CO2 by increased weathering at higher temp Decrease greenhouse – increase ppt of carbonates? Bob Berner’s calculations of changes in CO2 over the Phanerozoic Bob Berner’s calculations of changes in CO2 over the Phanerozoic “Hot” houses Bob Berner’s calculations of changes in CO2 over the Phanerozoic “Hot” houses “Ice” houses Fig. 8-18 Effect of humans • Pre-industrial – Steady state on decadal to century timescales – Ocean a net source of CO2 • Neutralizes river bicarb and oxidation of OM from rivers – Burial of org C • That which escaped oxidation and marine OM • Humans – Oceans a sink for CO2 – Increase sediment and nutrient load to rivers/ocean – Eutrophication, hypoxia, denitrification Fig. 10-16 A portion of the biogeochemical cycles of inorganic carbon (Cing) and organic carbon (Corg), nutrient N and P, and suspended solids (SS) in the land–ocean system. (a) Geological, long-term system; (b) one possible situation today. In (b), the fluxes of organic and inorganic carbon and suspended solids to the seafloor are increased over their pristine geological values in (a). These increases are due to human activities. Notice the net heterotrophic nature of the ocean giving rise to a net flux of carbon dioxide to the atmosphere prior to human interference in the carbon cycle. Now more carbon dioxide enters the ocean because of the burning of fossil fuels and deforestation practices (see Chapter 12). Fluxes are in millions of tons of C, N, P, and suspended solids per year. (After Wollast and Mackenzie, 1989.) CO2 All fluxes are millions of tons of C per year 460 (net) oxidation Corg (terr.) 360 200 400 Riverine inputs Cing 100 (net; approx. 50,000 (r) - 50,100(pp)) The Ocean 400 rxn. (1) Corg (marine +terr) Burial in sediments Cing 140 200 rxn. (1) Ca 2 2 HCO 3 CaCO 3 CO 2 H 2 O Fossil fuel burning • Transferring large amounts of CO2 from rock cycle to atm with no equivalent rapid uptake mechanisms! – Ocean uptake limited by the biological pump (nuts) – Uptake by terr. systems not rapid enough – Accumulates in atm • How does increase affect climate? – Depends on time scales of increase in atm conc versus time scales of changes in earth’s heat balance (via its circulatory system) – Positive and negative feedback responses N and P Cycles 80 y-1 Global nitrogen reservoirs, fluxes and turnover times. Major reservoirs are underlined, pool sizes and fluxes are given in Tg (1012 g) N and Tg N yr-1. Turnover times (reservoir divided by largest flux to or from reservoir ) are in parentheses. Atmosphere 120 (NF) 98 (DN) 121 (NF) 172 (DN) Land 27 (RT) Oceans The Pre-Industrial N Cycle (fluxes = Tg N/yr) (1860’s numbers from Galloway et al., 2004) Greenhouse effect Ice volume Global sea level Atmospheric CO2 (+) Oceanic primary productivity Area of continental shelves Shelf denitrification Oceanic fixed-N inventory Stimulates N-fixation Fig. 14-13 The iron fertilization hypothesis for the intensification of the biological pump during glaciations. Stratospheric Effects Atmosphere PM & Visibility Effects Ozone Effects NOx Energy Production Food Production NOx NHx NH3 Agroecosystem Effects Crop People (Food; Fiber) Human Activities The Nitrogen Cascade --Indicates denitrification potential Animal Soil Norg Terrestrial Ecosystems GH Effects N2O Forests & Grassland Soil NO3 N2O Groundwater Effects Surface water Effects Coastal Effects Ocean Effects Aquatic Ecosystems (t≈ 100 yr) 3.4 x (14 to 32) = 50-110 Retained in soils or denitrified Anthro. N fixation = 140 Tg N/yr - 41 - 8.5 -9 -3.4 61.9 Tg N/yr ~80 Tg N/yr missing ? (?) Nr and the Atmosphere NOx emissions contribute to OH, which defines the oxidizing capacity of the atmosphere NOx emissions are responsible for tens of thousands of excess-deaths per year in the United States O3 and N2O contribute to atmospheric warming N2O emissions contribute to stratospheric O3 depletion Nr and Freshwater Ecosystems • Surface water acidification – Tens of thousands of lakes and streams – Biodiversity losses • As reductions in SO2 emissions continue, Nr deposition becomes more important. Nr and Coastal Ecosystems • Increased algal productivity • Shifts in community structure • Harmful algal blooms • Degradation of seagrass and algal beds • Formation of nuisance algal mats • Coral reef destruction • Increased oxygen demand and hypoxia • Increased nitrous oxide (greenhouse gas) Sybil Seitzinger, 2003 There are significant effects of Nr accumulation within each reservoir These effects are linked temporally and biogeochemically in the Nitrogen Cascade Atmosphere Terrestrial Ecosystems Food Production NHx Agroecosystem Effects Crop People (Food; Fiber) Animal Soil Norg Human Activities The Nitrogen Cascade Galloway et al., 2003a Aquatic Ecosystems Atmosphere PM & Visibility Effects Food Production NHx NH3 Agroecosystem Effects Crop People (Food; Fiber) Human Activities The Nitrogen Cascade Galloway et al., 2003a Animal Soil Terrestrial Ecosystems Forests & Grassland Soil Norg Groundwater Effects Surface water Effects Coastal Effects Ocean Effects Aquatic Ecosystems Atmosphere PM & Visibility Effects Food Production NHx NH3 Agroecosystem Effects Crop People (Food; Fiber) Human Activities The Nitrogen Cascade Galloway et al., 2003a Animal Soil Norg Terrestrial Ecosystems Forests & Grassland Soil NO3 Groundwater Effects Surface water Effects Coastal Effects Ocean Effects Aquatic Ecosystems Atmosphere PM & Visibility Effects Ozone Effects NOx Energy Production Food Production NOx NHx NH3 Agroecosystem Effects Crop People (Food; Fiber) Human Activities The Nitrogen Cascade Galloway et al., 2003a Animal Soil Norg Terrestrial Ecosystems Forests & Grassland Soil NO3 Groundwater Effects Surface water Effects Coastal Effects Ocean Effects Aquatic Ecosystems Atmosphere PM & Visibility Effects Ozone Effects NOx Energy Production Food Production NOx NHx NH3 Agroecosystem Effects Crop People (Food; Fiber) Human Activities The Nitrogen Cascade --Indicates denitrification potential Animal Soil Norg Terrestrial Ecosystems Forests & Grassland Soil NO3 Groundwater Effects Surface water Effects Coastal Effects Ocean Effects Aquatic Ecosystems Stratospheric Effects Atmosphere PM & Visibility Effects Ozone Effects NOx Energy Production Food Production NOx NHx NH3 Agroecosystem Effects Crop People (Food; Fiber) Human Activities The Nitrogen Cascade --Indicates denitrification potential Animal Soil Norg Terrestrial Ecosystems GH Effects N2O Forests & Grassland Soil NO3 N2O Groundwater Effects Surface water Effects Coastal Effects Ocean Effects Aquatic Ecosystems Ind. N fix. Population Total react. N Crop N fix. Fossil fuel N F Fig. 10-16 A portion of the biogeochemical cycles of inorganic carbon (Cing) and organic carbon (Corg), nutrient N and P, and suspended solids (SS) in the land–ocean system. (a) Geological, long-term system; (b) one possible situation today. In (b), the fluxes of organic and inorganic carbon and suspended solids to the seafloor are increased over their pristine geological values in (a). These increases are due to human activities. Notice the net heterotrophic nature of the ocean giving rise to a net flux of carbon dioxide to the atmosphere prior to human interference in the carbon cycle. Now more carbon dioxide enters the ocean because of the burning of fossil fuels and deforestation practices (see Chapter 12). Fluxes are in millions of tons of C, N, P, and suspended solids per year. (After Wollast and Mackenzie, 1989.) Sulfur cycle Nr and Terrestrial Ecosystems • N is the limiting nutrient in most temperate and polar ecosystems • Nr deposition increases and then decreases forest and grassland productivity • Nr additions probably decrease biodiversity across the entire range of deposition Sulfate Pyrite uplift and weathering Pyrite burial Hydrothermal uptake 2 Fe 2 O 3 16 Ca 2 16 HCO 3 2 8 SO 4 4 FeS 2 16 CaCO 3 8 H 2 O 15 O 2 CO 2 H 2 O CH 2 O O 2 2 Fe 2 O 3 16 Ca 2 16 HCO 3 2 8 SO 4 4 FeS 2 16 CaCO 3 8 H 2 O 15 O 2 CO 2 H 2 O CH 2 O O 2 2 Fe 2 O 3 16 Ca 2 16 HCO 3 2 8 SO 4 4 FeS 2 16 CaCO 3 8 H 2 O 15 O 2 Sulfate SO2 DMS H2S H2S SO2 Sulfate SO2 SO2 DMS H2S H2S SO2 William Turner, “The fighting Téméraire tugged to her last berth to be broken up” (Tambora) Ash and debris from volcanic eruptions Edvard Munch “The Scream” (possibly inspired by Krakatoa) The CLAW Hypothesis (neg. feedback; reflectivity) Clouds Temp. (+) ? (+/-) Cloud condensation nuclei (+) DMS (+) Plankton Fig. 14-18 Fig. 14-19