FWJvR – ILSI -SA – Kenya 23-24 June 2014 – Copy
advertisement

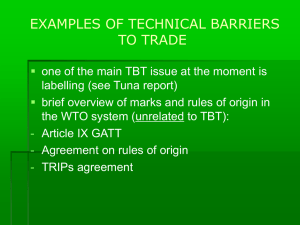
TM Developing harmonized food safety regulations and standards based on science to promote regional and international trade Wilna Jansen van Rijssen PhD, ILSI-SA, Dr Boishoko Ntshabele, Director: Food Safety and Quality Assurance, Department of Agriculture, Forestry and Fisheries, SA 24 June 2014 Globalization of trade in foods • Advantages: – Consumer choices of products – Foreign exchange • Impediment: – Tariff and non-tariff barriers to trade TM International organizations • Codex Alimentarius Commission (FAO/WHO) - 1962 – Protect health of consumers – Ensure fair practices in trade • World Trade Organization (WTO) Agreements (1995) – SPS (Sanitary and Phyto-Sanitary) - measures – TBT (Technical Barrier to Trade) - measures TM GOALS OF THE AGREEMENT • Recognise the sovereign right of members to provide the level of health they deem appropriate • Ensure that SPS measures do not represent unnecessary , arbitrary, scientifically unjustifiable, or disguised restriction on international trade. TM REGIONAL AND INTERNATIONAL TRADE SPS AGREEMENT This Agreement applies to all sanitary and phytosanitary measures which may, directly or indirectly, affect international trade. Such measures shall be developed and applied in accordance with the provisions of this Agreement. http://www.wto.org/english/res_e/publications_e/wtocan_e.pdf 5 TM SPS MEASURES • Protect animal and plant life or health within the territory of the Member • Protect human and animal health from risks arising from additives, contaminant, toxins, disease-causing organisms in foods, beverages or feedstuffs • Diseases carried by animals, plants or products thereof • Spread of pest TM SPS vs TBT • SPS – a narrowly defined range of health protection but strict requirements on these measures. Always based on scientific principles • TBT – range of technical requirements – to avoid consumer deception, standards, quality, some safety and health. TM WTO: SBS AND TBT AGREEMENTS (WHO/FSF/FOS/97.8.REV 1 (1998) TM SCIENTIFIC JUSTIFICATION • An appropriate level of protection (ALOP) or acceptable level of risk? • How does members show that measures are based on science? TM HOW? International standards HARMONIZATION and/or Scientific risk assessment consistently applied / not more trade restrictive than necessary TM REGIONAL AND INTERNATIONAL TRADE To harmonize sanitary and phytosanitary measures on as wide a basis as possible, Members shall base their sanitary or phytosanitary measures on international standards, guidelines or recommendations, where they exist, except as otherwise provided for in this Agreement 11 TM INTERNATIONAL ORGANIZATIONS • Codex Alimentarius Commission (CAC) - Food • International Office of epizootics (OIE) animals • International Plant Protection Convention (IPPC) - Plants TM REGIONAL AND INTERNATIONAL TRADE 13 TM CASE STUDY Steviol glycoside • Stevia rebaudiana Bertoni leaves • Sweetener • Stevioside and Rebaudioside A (Nine named steviol glycosides) • ADI of 0-4 Mg/kg in 2004 • European Commission authorized the use in 2011 http://whqlibdoc.who.int/publications/2009/9789241660600_eng.pdf TM SPS MEASURES SISTERS NOT BASED ON 3 • Available scientific evidence • Relevant processes and production methods • Relevant inspection, sampling and testing protocols • ETC • Economic factors TM CODEX: STRUCTURE OF RISK ANALYSIS Scientific advice / Information/ analysis Risk Assessment Risk Management Regulation and control/ Enforcement Risk Communication Risk assessment policy TM COMPONENTS OF RISK ANALYSIS AS DEFINED BY CODEX Risk assessment: A scientifically based process consisting of the following steps: i) hazard identification; ii) hazard characterization; iii) exposure assessment and iv) risk characterization. http://www.codexalimentarius.org/scientific-basis-forcodex/en/loading 18 TM COMPONENTS OF RISK ANALYSIS AS DEFINED BY CODEX Risk management: The process, distinct from risk assessment, of weighing policy alternatives in consultation with all interested parties, considering risk assessment and other factors relevant for the health protection of consumers and for the promotion of fair trade practices, and, if needed, selecting appropriate prevention and control options. 19 TM COMPONENTS OF RISK ANALYSIS AS DEFINED BY CODEX Risk communication: An interactive process of exchange of information and opinion on risk among risk assessors, risk managers and other interested parties.(individuals, groups and institutions). 20 TM FOOD SAFETY REGULATION: PROACTIVE LEGISLATION / REACTIVE LEGISLATION • RISK ASSESSMENT : toxicity assessment Registration/permit for new molecules (additives /pesticides/toxicants/vet drugs) • Hazard assessment/characterization: Toxicological assessment of chemical ADI / ARfD (acute reference dose)/PMTDI (provisional maximum tolerate daily intake) TM FOOD SAFETY REGULATION: EXPOSURE ASSESSMENT: Residue limits (MRLs) /tolerance levels , statutory determined/GAPs/exposure Dietary intake data • GEMS/food intake (Global Environmental Monitoring System- Food Contamination Monitoring and Assessment Programme http://www.who.int/foodsafety/chem/acute_data/en/ http://www.who.int/foodsafety/chem/cluster_diets_2012.pdf http://www.who.int/foodsafety/publications/chem/regional_diets/en/ • Map - Countries by Cluster – 2006 png, 151kb TM JECFA assessment CASSAVA toxicants 24 TM PROVISIONAL MEASURES INSUFFICIENT scientific evidence to permit a final decision. • Emergency response • Provisional measures adopted on the basis of available pertinent info • Seek for additional information • Reasonable time period TM PRECAUTIONARY PRINCIPLE • Precaution vs precautionary principle (approach) • Cartagena Protocol on Biosafety • Risk management - decision making • “uncertainty” in risk assessment • No zero risk TM South Africa - additives • • • • • • • • • • • • TM List of permissible sweeteners referred to in Regulation 4 of the regulations relating to the use of sweeteners in foodstuffs Regulations relating to the use of sweeteners in foodstuffs (R733/201) Also see Codex General Standards for Food Additives List of permissible sweeteners referred to in Regulation 4 of the regulations relating to the use of sweeteners in foodstuffs Regulations relating to baking powder and chemical leavening substances (R2486/1990) Regulations – Preservatives and antioxidants: Amendment (R60/2009) Regulations relating to mayonnaise and other salad dressings (R92/1986) Regulations relating to food colourants (R1055/1996) Regulations – Jam, conserve, marmalade and jelly (R2627/1986) Regulations governing emulsifiers, stabilisers and thickeners and the amounts thereof that foodstuffs may contain (R2527/1987) Regulations - Preservatives and antioxidants (R965/1977) Regulations governing acids, bases and salts and the amounts thereof the foodstuffs may contain (R115/1986) REGIONAL AND INTERNATIONAL TRADE • Members shall accept the sanitary or phytosanitary measures of other Members as equivalent, even if these measures differ from their own or from those used by other Members trading in the same product, if the exporting Member objectively demonstrates to the importing Member that its measures achieve the importing Member's appropriate level of sanitary or phytosanitary protection. • • • • Zoning (Kruger National Park – TB in lions) Compartmentalization (a farm quarantined) Zero tolerance Single source (a single exporting a novel product) 28 TM EQUIVALENCE • More than one measure may be effective • The importer must be granted reasonable access to inspect, test etc • Ex – FMD treatment of milk (EU), New Zealand accept products only from FMD free countries. • Pest and disease free regions – fruit (oranges) TM DISPUTE SETTLEMENTS • Mad cow disease • Animal growth promoter - 3x naturalhormones & 3 synthetic hormones • EU ban on GMOs not based on science, consumer perceptions TM WTO: RIGHTS AND OBLIGATIONS • Transparency- Information exchange – One enquiry point – Publication of regulations – Notification procedures • International harmonization (Codex standards) – Food additives/ veterinary drugs/pesticide residues • Risk-based approach – HACCP (Hazard Analysis and Critical Control Point), process control – Risk assessment • TM Control, inspection an approval procedures CHALLENGES • Harmonizing – Guidelines for pesticides and veterinary drugs risk assessment and management. • Capacity • Toxicologist? TM TM WTO. http://www.wto.org wilnajvr@telkomsa.net