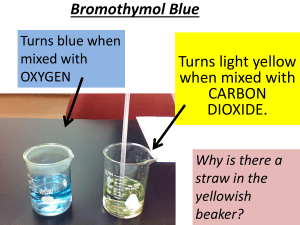
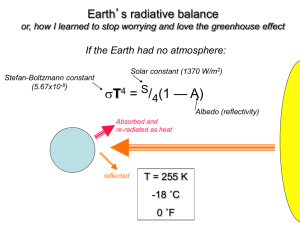
MS PowerPoint
advertisement

Course on Carbon dioxide to Chemicals and Fuels PRESENTATION - FIVE 24TH February 2014 On Line Course of NCCR (Total Number of Projections for this Lecture is 20) NCCR on Line L3 1 PHOTOELECTROREDUCTION OF CO2 Appealing Approach! An important energy input contribution from light might be expected, thus diminishing electricity consumption Principle An Example J.P. Collin & J.P. Sauvage, Coord. Chem. Rev. 93 (1989) 245 A study on photo-electro-reduction of CO2 Possible Mechanistic Route By insitu-IR Photovoltomogram, λ= 560 nm (0.5 mW \cm2) J, O‘M. Bockris & J. C. Wass, Mater Chem Phys, 22 (1989) 249 Study on photo-electro-reduction of CO2 J, O‘M. Bockris & J. C. Wass, Mater Chem Phys, 22 (1989) 249 Metal islet catalysts deposited on a p-CdTe electrode in DMF-0.1 M TEAP/5% H20 Product analysis results for CO2 reduction on phthalocyanine/p-CdTe MPc catalysts adsorbed on a p-CdTe electrode in DMF-0.1 M TEAP/5% H20 Study on Photo-electro-reduction of CO2 Current-potential curves for trinuclear carbonyl catalysts adsorbed on a p-CdTe electrode in DMF-0.1 M TEAP/5% H20. Product analysis results for CO2 reduction on carbonyl/p-CdTc Iron carbonyl is the best among the three carbonyls studied J, O‘M. Bockris & J. C. Wass, Mater Chem Phys, 22 (1989) 249 Study on photo-electro-reduction of CO2 Current-potential curves for crown ether Product analysis results catalysts added to the electrolyte for a p-CdTe J, O‘M. Bockris & J. C. Wass, Mater Chem electrode in DMF-0.1 M TEAP/S% H20 Phys, 22 (1989) 249 Catalytic shift (ΔE) Catalytic shift (ΔE) times the CO faradaic efficiency for metal catalysts on p-CdTe as a function of M-O bond energy ΔE values for CO production are linear For metal-phthalocyanine catalysts on pCdTe as a function of M-O bond energy J, O‘M. Bockris & J. C. Wass Mater Chem Phys, 22 (1989) 249 Catalytic shift (ΔE) J, O‘M. Bockris & J. C. Wass, Mater Chem Phys, 22 (1989) 249 For trinuclear carbonyl catalysts on p-CdTe as a function of M-C bond energy CARBON MANAGEMENT • Fertilization of open waters to increase primary production & hence to absorb more carbon in fixed form • Disposal of captured carbon dioxide directly into oceanic waters • Injection of captured CO2 into sub-seabed geological formations CO2 sequestration Barriers to wider implementation • High cost of capturing, processing, & transporting anthropogenic CO2 • Incomplete understanding of reservoir processes • Underdeveloped monitoring & verification technologies • Unclear emissions trading regulations • Potential conflicts of interest between sequestration & EOR or natural gas recovery CO2 Sequestration Public Perception The technology is in its infancy and unproven • The technology is too costly • Not enough is known about the long-term storage of CO2 • The capture and storage of CO2 are seen as being energy intensive • The option presents an enormous engineering and infrastructure challenge • It is not a long-term solution Barriers can only be overcome by research and design & effective demonstration of the technology Perceptions: Large-Scale CO2 Utilization & Sequestration • Two big challenges – Reducing costs – Developing storage Reservoirs • Utilization scores on these two challenges but opportunities are limited • Utilization will play a major role in initial sequestration • Utilization will play a minor role for long term large scale sequestration UTILIZATION • Opportunities – Help economics – Estimates storage issues Why large scale use of CO2 such a challenge? – Market sizes – Transportation costs – Product life times – Energy considerations TRANSPORTATION COSTS • Many production sources • CO2 expensive to transport well in small quantities. • Use sources of opportunities (process by products natural wells) • Example –US 1997 capacity for liquid CO2 – 9.7 million metric tons – 93 plants – Largest 900 metric tons/day – Average 300 metric tons /day WHAT HAS BEEN COVERED SO FAR The electronic structure of Carbon dioxide M. A. Scibioh & B. Viswanathan,Proc. Indn. Natl. Acad. Sci., 70 A (3), 2004.407-462 CHEMICAL REDUCTION OF CARBONDIOXIDE ADDING HYDROGEN AND ELIMINATING WATER Electrochemical Reduction of CO2 The possible electrochemical Reactions and the corresponding potentials H2O to H2(g)+ 0.5O2(g) CO2 + H2 to HCOOH CO2 + H2O to HCOOH + 0.5O2 CO2 + H2 to CO + H2O CO2 to CO + 0.5O2 CO2 + 3H2 to CH3OH + H2O ` CO2 + 4H2 to CH3OH + 2 H2O CO2 + 2 H2O to CH3OH + 1.5O2 CO2 + 2 H2O to CH4 + 2 O2 E0 1.23 1.34 1.33 1.20 1.06 Delta G0 (Kcal/mol) 56.7 5.1 61.8 4.6 61.3 -4.1 -31.3 166 195 Table : Sector-wise contribution of CO2 emissions Sector Percent Contribution Land use and forestry 17 Industry Residential and commercial Buildings Transportation Power waste and waste water 19 8 13 26 3 Scheme.1.Chemical Transformations of CO2 Barriers for Further Progress (1) the magnitude of environmental consequences, (2) the economic costs of these consequences, (3) options available that could help avoid or diminish the damage to our environment and the economy (4) the environmental and economic consequences for each of these options (5) an estimate of cost for developing the technology to implement these options and (6) a complete energy balance which accounts for energy demanding steps and their costs. Suggested Some References 1. A Beher, Carbon Dioxide Activation by Metal Complexes VCH, Weinheim (1988) 2. Catalytic Activation of Carbon Dioxide (ACS Symp Ser) (1988) 363 3. M. Aulice Scibioh and V.R. Vijayaraghavan, J. Sci. Indus. Res., 1998, 57, 111-123. 4. M. Aulice Scibioh and B. Viswanathan, Proc. Indn. Natl. Acad.Sci., 70 A (3), 2004, 407-462 5. M. Aulice Scibioh and B. Viswanathan, Editor. Satoshi Kaneco, Japan, Photo/ Electrochemistry and Photobiology for Environment,Energy and Fuel, 2002, 1- 46, ISBN: 81-7736-101-5. 6. F. Bertilsson and H. T. Karlsson, Energy Convers. Mgmt Vol. 37,No. 12, pp. 1725-1731, 1996 7. I. Omae, Catalysis Today 115 (2006) 3352 8. M. Gattrell, N. Gupta and A. Co, J. Electroanal Chem, 594, (2006),1-19. 9. Enzymatic and Model Carboxylation and Reduction Reaction for Carbon Dixoide Utilization (NATO ASF Ser C 314 (1990) 10. Electrochemical and Electrocatalytic Reaction of Carbon Dioxide (Eds B P Sullivan, K Krist and H E Guard) Elsevier Amsterdam (1993) 11. M M Halmann Chemical Fixation of Carbon Dixoide CRC Boca Raton (1993) D Walther Coord Chem Rev 79 (1987) 135. 12. P. G. Jessop, F. Jo, C-C Tai, Coordination Chemistry Reviews 248 (2004) 2425-2442 THE TOPIC THAT FOLLOWS IS DRY REFORMING OF CARBON DIOXIDE Topics in Reforming of Carbon dioxide (1) what is this reaction and why this reaction? (2)what are the concurrent possible reactions? (3) The basic thermodynamics of the reaction (4) Catalyst systems that have been studied. (5) The rationale for the selection of catalysts