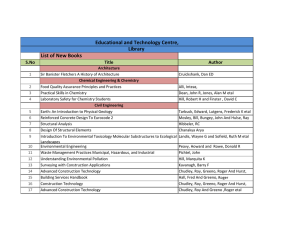
performance index
advertisement

CREB & The Development Of Novel Memory Enhancers Tim Tully Chief Science Officer Dart Neuroscience LLC Long-Term Memory In The Fruit Fly PERFORMANCE INDEX 100 80 60 10x spaced 40 10x massed 20 1x 0 0 1 2 3 4 5 RETENTION TIME (day) 6 7 Tully et al (1994) CREB Repressor Blocks LTM 100 PERFORMANCE INDEX 80 60 spaced 40 20 0 hs-dCREB2-r massed -20 1 3 5 7 TIME (days) Yin et al (1994) See also: Bourtchouladze et al (1994) CREB Activator Enhances Memory 7-DAY MEMORY 50 control CREB-a 40 30 20 10 0 1x 10x spaced TRAINING SESSIONS Yin etal. (1995) dCREB2 Homologs GENESEER Olson etal (2005) Drug Screen For Enhancers of CREB forskolin neuroblastoma cell ATP AC Ca/CaM cAMP PKA HT-0712 PDE AMP nucleus CREB CRE luciferase Memory Enhancement In Normal, Young Mice Vehicle 60 0.1mg/kg HT0712 (60’ after) Memory Score 50 40 30 * 20 10 8 8 8 8 0 2 Trials 5 Trials Paired-Associate Memory In Elderly Macaque 30 Training Days to Criterion 25 20 15 10 5 0 vehicle 1 mg/kg 10 mg/kg 100 mg/kg Mouse Model Of Rubinstein-Taybi Syndrome wild-type (sib) CBP+/- mice Oike etal (1999) Facilitation Of LTM In RTS Model 15-min Training 80 wt 70 CBP+ / - Memory Score 60 50 40 30 * 20 10 0 -10 Ve hic le HT0712 (0 .1 m g /kg ) Bourtchouladze etal (2003) Facilitation Of Rehabilitation After Stroke Post-stroke motor recovery 1.5 vehicle 1.0 HT-0712 (0.15 mg/kg ) 0.5 0 0 1 2 3 4 5 6 7 8 9 10 11 Day MacDonald et al. (2007) Neurorehabil. Neural Repair 6: 486. Cognitive Rehabilitation After Traumatic Brain Injury Hallam etal (2010) unpublished. Cognitive Rehab Generalizes Hallam etal (2010) unpublished. Clinical Safety Phase 1 (single rising dose) • 42 healthy young and elderly exposed (405 mg max dose) • no serious adverse events Phase 1 (repeat dose) • 24 healthy young and elderly exposed (135 mg max dose) • no serious adverse events Phase 1 (interaction with warfarin) • 21 elderly exposed (45 mg dose) • no serious adverse events Phase 2a (28 daily doses) • 55 elderly with AAMI (90 mg max dose) • no serious adverse events Drug Development Pipeline Project PDE4 PreClinical Research GABA-A Novel GLYT1 Phase II Phase III HT-0712 BackUp HT-2157 GalR3 MAO-B Phase I BackUp HT-1067 Back-ups HT-4313 Back-ups Hits Screening Hits Screening Acknowlegements Collaborators Ken Johns Phil Perera Anne Danks Alan Kaplan Tim Tully CEO CMO Director, Preclinical Development Director, Chemistry Founder, x-CSO Lila Davachi Emmanuel DeCamp TJU Jeff Kleim NYU UF-Gainesville HT-0712 Facilitates Motor Rehab After TBI Hallam etal (2010) unpublished. HT-0712 Facilitates Cognitive Rehab After TBI Hallam etal (2010) unpublished. Drug Alone Does Not Facilitate Hallam etal (2010) unpublished. No Measures Of Long-Term Memory In The Clinic… MEMORY RETENTION clinical measures of dementia STM observed ARM LTM MTM 0 1 3 TIME (hr) 5 24 7-Day WordRecall In Humans With AAMI P = 0.22 P = 0.03 P = 0.32 P = 0.20 7d - 7hr 0 -10 -20 -30 0 15 45 Dose (mg) 60 90