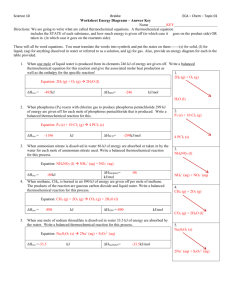
thermochemical equation
advertisement

THE UNIVERSITY OF QUEENSLAND Foundation Year THERMOCHEMISTRY II Lesson Overview Thermochemistry Heat Capacity Specific heat capacity Endothermic and Exothermic Equations Calorimetry Thermochemical Equations Heats of Changes of State Hess’s Law Standard Heats of Formation Thermochemical Equations 1 • A thermochemical equation is one that includes energy changes. • In exothermic reactions, heat is a product (it's being formed), so a reaction of this kind might look like this: A + B ---> C + D + heat • And similarly, if a reaction is endo, then it acts like a reactant (goes on the left side): A + B + heat ---> C + D Thermochemical Equations 2 • There are two ways to write a thermochemical equation: • 2 C2H6(g) + 7 O2(g) 4 CO2(g) + 6 H2O(g) H = -2855 kJ or • 2 C2H6(g) + 7 O2(g) 4 CO2(g) + 6 H2O(g) + 2855 kJ • Are these reactions exothermic or endothermic? Thermochemical Equations 3 • For endothermic reactions, energy must be added to the reactants to make it happen. • Heat may be considered as a reactant. • 2 NH3(g) + 92 kJ N2(g) + 3 H2(g) or • 2 NH3(g) N2(g) + 3 H2(g) H = +92 kJ • When writing thermochemical equations, the state symbols must be included. This is because changing the state of a Heat and Changes of State (1) • All solids absorb heat in melting to liquids. The heat absorbed by one mole of a substance in melting from a solid to a liquid at a constant temperature is called the molar heat of fusion (Hfus.). • The heat lost when one mole of a liquid changes to a solid at a constant temperature is the molar heat of solidification (Hsolid. ) Heat and Changes of State (2) • The amount of heat absorbed by one mole of a liquid that is undergoing evaporation is called the molar heat of vaporisation. ( Hvap) • The condensation of 1 mole of vapour releases heat as the molar heat of condensation (Hcond). Vapour Hcond = -ve Hvap = +ve Enthalpy Liquid Hsolid = -ve Hfus = +ve Solid Heat of Solution • Heat changes can occur when a substance is dissolved in a solvent. The heat change caused by dissolution of one mole of substance is the molar heat of solution, Hsoln. Exothermic Solvation http://wine1.sb.fsu.edu/chm1046/notes/SolnProp/SolnProc/Hsolv1gif.gif Endothermic Solvation http://wine1.sb.fsu.edu/chm1046/notes/SolnProp/SolnProc/SolnProc.htm http://www.wou.edu/las/physci/ch412/heatsoln.gif Thermochemical Equation Calculations • Thermochemical equations obey several simple rules that make computation of the enthalpy change in a reaction easy. 1. The magnitude of H is directly proportional to the amount of reactants used in the reaction 2. H for a reaction is equal in magnitude but opposite in sign to the reverse reaction. 3. Hess' Law: The value of H for a reaction is the same no matter what path is used to get from reactants to products. Hess’s Law of Summation • Hess's Law of Heat Summation states: If you add two or more thermochemical equations to give a final equation, then you can also add the heat changes to give the final heat changes. Hess’s Law of Summation(2) To find the enthalpy for: • C(s, diamond) C(s, graphite) • C(s, graphite)+ O2(g) CO2(g) H = -393.5kJ • C(s, diamond)+ O2(g) CO2(g) H =-395.4kJ Write the reverse of equation (a) to give • CO2(g) C(s, graphite)+O2(g) H = +393.5kJ Hess’s Law of Summation(3) • Adding the equations should give us our original equation. • Now do the same thing with the enthalpy values :i.e.: H = -395.4kJ + H = +393.5kJ H = -1.9kJ Heats of Formation • The standard heat of formation (Hf) of a compound is the change in enthalpy that accompanies the formation of one mole of the compound from its elements with all substances in their standard states at 25oC. • The H for a reaction is the difference between the standard heats of formation of all the reactants and products. • ie: H = Hf(products) - Hf(reactants) http://cwx.prenhall.com/petrucci/medialib/media_portfolio/text_images/FG07_16.JPG http://cwx.prenhall.com/petrucci/medialib/media_portfolio/text_images/TB07_02.JPG References 1. 2. 3. 4. 5. 6. http://www.chem.vt.edu/RVGS/ACT/notes/Chap_8_Trip tik.html http://apchem.virtualave.net/concepts/thermochem.html http://chemed.chem.purdue.edu/demos/movies/small_mo vies/5.2small.mov http://www.wou.edu/las/physci/ch412/heatsoln.gif http://wine1.sb.fsu.edu/chm1046/notes/SolnProp/SolnPr oc/Hsolv1gif.gif http://cwx.prenhall.com/petrucci/medialib/media_portfol io/text_images/ End of Lecture