Chemistry Extra Credit Labs
advertisement

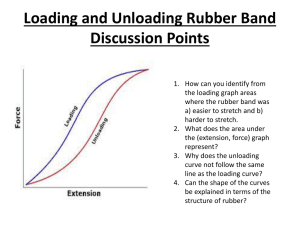
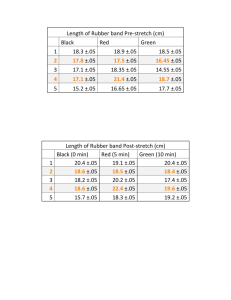
Chemistry Extra Credit Labs Alison Parrott Mitchell Loop La Salle Period 6 April 9, 2012 Surfactants • Purpose – The purpose of this lab is to observe an unusual surface property of water that results from hydrogen bonding using water and oil. • Materials – Bowl – Water – Paper clip – Rubber band – Dropper – Vegetable oil – Liquid dish detergent Procedure 1. Thoroughly clean and dry the dish. 2. Fill the dish almost full with water. 3. Gently place the paper clip in the water and observe what happens. 4. Repeat steps one and two. 5. Gently place the open rubber band on the water 6. Slowly add oil drop by drop onto the water encircled by the rubber band until the water is covered with a layer of oil. Observe for fifteen seconds. 7. Allow one drop of dish detergent to fall onto the center of the oil layer. Observe for fifteen seconds. Analyze and Conclude • What happened to the paper clip in Step 3? Why? The paper clip sank to the bottom of the bowl. • If a paper clip becomes wet, does it float? Explain your answer? – No, the paper clip still sinks because the mass is great enough to break the surface of the water. • What shape did the rubber band take when the water inside it was covered with oil? Why did it take the observed shape? – The rubber band took the shape of the surrounding oil because the oil can float on top of the water because of the apparent hydrogen bonding within the water and the contrasting effects with the oil. • Describe what happened when dish detergent was dropped onto the layer of oil. – The soap remained solid while it sat on top of the oil layer. After some time, the detergent sank below this layer and dispersed itself throughout the water.