Training - Powerpoint
advertisement

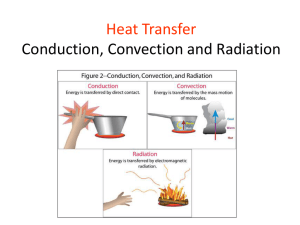
Conduction, Convection, & Radiation Vanderbilt Student Volunteers for Science Fall 2013 I. Introduction- What is temperature? What is heat? • Ask: what is temperature? – scientific measure of how hot or cold something is – measured with a thermometer – measure of the average kinetic energy of the molecules in a substance. • Ask: What happens when we add ice to hot tea? The ice melts. Energy from tea is transferred to the ice. This transfer of energy is called heat. 2. Liquid Crystal Temperature Sensors • Ask students if they have seen objects that change color with temperature. (mood rings, strip thermometers..) • Discuss the RULES for using the temperature sensors: – The sensor must not be placed in the sun or near a heat source – Always hold the strips using the clear laminated part – Do NOT touch the liquid crystal unless instructed to do so. • Hand out liquid crystal temperature sensors to each pair of students. 2A. Experiment • Tell students to place the temperature sensor on the white foam pad, black side up. • Tell 1 student/pair to place 3 fingertips on the liquid crystal sensor for 15 sec. Record what happens. • Observations: – color of the sensor changes. – color changes spread out from the center of the finger and changes color as it spreads. 2. Discussion • Ask : What color is in the center of the finger print? At the outer edge? What color fades first? Last? What color =coolest temp? – Black What color = warmest temp? – Blue • Order from cooler to warmer temperatures? Black, yellow, red, green, blue. • Explanation: The surrounding area’s colors were produced as a result of conduction of heat through the crystal. • Place strip back on the desk top to return to room temp. 2B. How do Liquid Crystals work? • Explain to students that liquid crystals are sensitive to temperature and will separate visible light into different colors according to the temperature of the crystal. Black is the coolest color. • Where does the thermal energy come from to change the liquid crystal color? – Answer: Your skin temperature is higher than room temperature. Heat flows from your fingers into the object. • Where does the energy go when the color changes back to its colder temperature? – Answer: It is transferred to the desk or Styrofoam pad. Heat flows from the warmer to the cold. 3. How is energy transferred? • Thermal energy is transferred by heat. – Heat always flows from a hotter object to one that is cooler. In this case, your body is warmer than room temperature and heat flows from your finger to the sensor. • Ask students if the know the different ways thermal energy is transferred? Conduction, convection, or radiation. • Tell students we are going to “see” conduction, convection and radiation by using the liquid crystal sensors. 3. How is energy transferred? A. Radiation • Radiation is the transfer of energy by electromagnetic waves. • Ask: Can you name some sources that transfer heat by radiation? – The sun, a fire, bar heater, incandescent light bulb …. • Radiation does not rely upon any contact between the heat source and the heated object. We can feel heat from the Sun or a fire even though we are not touching them. 3. Demonstration - Visualizing Radiation A VSVS member holds the large liquid crystal sensor up. Note the color. Hold the lamp in a vertical position, turn it on and hold the large sensor vertically, about 10 inches away. Wait until the sensor changes color, then remove the light and observe changes. Optional Demonstration: Visualizing Sun Radiation (if the sun is shining into the classroom) Now hold the sensor in the sunlight and note the color changes. Point out that there is no contact between the lamp and the sensor. 3. Experiment – Visualizing Convection Define convection and ask: Can you name some sources that transfer heat by convection? Heating water, hot air balloons… Experiment: 1. Show how to activate the hot pack and place on the white foam board. 2. Tell students to hold their hands above it, to the side, and below it to feel convection 3. Hold the liquid crystal sensor 2 cm above the heat pack (DO NOT TOUCH IT) and note color changes. Explanation: When the heat pack warms the air next to it (through conduction). The warm air molecules move more quickly, becomes less dense, and rises. The sensor changes color. C. Conduction • Define: Conduction is the transfer of thermal energy between objects that are touching. • Ask: Can you name a good and poor thermal conductor? Metals are usually good conductors. Foam boards are not. Experiment: Visualizing Conduction • • Hand out the thermal conductivity boards. Ask students if they can identify the 3 different materials? Copper, wood and iron. • Tell students to place the heat pack at the lowest edge of the 3 exposed prongs. – Wait a few minutes and observe changes in liquid crystal colors. • Record the observations. – The color change starts nearest the heat pad, travels up the materials. – The rates are different for the 3 materials. • What metal had the fastest changing temperature sensor? Copper – Slowest? Wood – What is the best thermal conductor? Copper Experiment 2. Observing Ice melting Distribute pairs of black blocks Place some ice in the middle of each block and tell students to observe. Do not tell them what the blocks are made of. Pass out the plastic bags with wood, styrofoam and metal squares and thermometer strips. Spread on long piece of foam board. Tell the students to: 1. Place a palm on top of each block (no more than 1 sec). 2. Put the blocks in order from coolest to warmest, record results on the board (aluminum, wood, foam). 3. Set aside the squares so that they can return to room temperature. Experiment 3. Ice Melting on 3 Different Materials. • Show the students a strip thermometer and explain that it is made of the same liquid crystal material, with a temperature scale added. – The dark blue color denotes the room temperature • Tell students to place a strip on wood block and record temperature. Repeat with the Styrofoam and metal blocks. • Ask students if the temperatures of the blocks are different. (Should be no significant difference within a group) Experiment 3. Ice Melting on 3 Different Materials cont. • Ask: What will happen to ice if placed on the blocks? • Tell students to place a small piece of ice in the middle of each block and record results. The ice on the metal will melt in just a few seconds. • Wipe ice off the metal block and measure temperature again. • Ask: Why did the ice on the metal melted faster? Metal is a good conductor, it transfers heat from it to the ice faster than the other 2 materials. Results Ask: Why did the metal square felt cooler than the others, when the actual temperature is the same? The cold square is CONDUCTING heat away from hand so it is actually your hand that is cooling. Ask students which object is the best conductor? The metal square is the best conductor. Ask students which object would be a good insulator? Styrofoam. CLEAN UP • • • Wipe off wet surfaces. Place all materials back in kit. Discard leftover ice at the school if possible. Return kit immediately. It is needed by another group the following day. Radiation Extension Radiation extension: A VSVS member can cast a shadow on one side of the large sensor with a book. This blocks the radiation reaching half the sensor . Expose the sensor to the lamp or to the sun for a few minutes and note the color changes. Students will see that the sun’s radiation does not travel through the book. Ask students how this could relate to weather on a cloudy day? The sensor can also be held at an angle to the lamp’s or sun’s rays rather that facing it directly. Note the sensor warms up slower. Ask students how this can relate to the seasons.