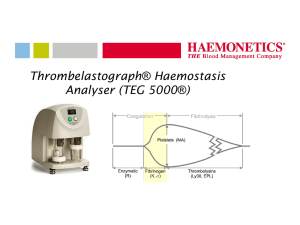
CBER 510(k) Challenges and Strategies
advertisement

CBER 510(k) Challenges and Strategies Susan Finneran Director of Clinical and Regulatory Affairs Background on Haemonetics NYSE traded company 2,070 employees world wide Based in Braintree, MA. Hospital and blood collection customers in more than 80 countries Vision: To be the Global Leader in Blood Management Solutions 2 Copyright © 2009 Haemonetics Corp. We have a growing portfolio of customer solutions Hospital Blood Center Recruitment & Interview Blood Collection Processing & Testing Inventory & Distribution Hospital BB Transfusion Inventory Preparation Intra Post Point of Care Blood Use Optimization Reports Business Design InSight™ Model Dashboards Pre Information Management Dashboards TEG® cardioPAT Cell Saver® MCS+ Cymbal® Donor Recruitment Programs PCS2 ACP®215 Automation Nation™ OrthoPAT® Devices Blood Collections Optimization Consulting Services Business Solutions Lean & Six Sigma Blood Use Optimization Consulting Services Services 3 3 ® Copyright © 2009 Haemonetics Corp. Haemonetics Devices Automated Apheresis Devices Equipment, imbedded software and disposable sets Submitted to CBER 510(k) with clinical studies Recently down classified (Class II) Autotransfusion Devices Equipment, imbedded software and disposable sets Submitted to CDRH Class II Laboratory Studies 4 Copyright © 2009 Haemonetics Corp. Types of 510ks submitted to CBER Traditional – 90% Special -10% STED- none Abbreviated- none Third Party- not eligible for CBER devices 5 Copyright © 2009 Haemonetics Corp. Premarket Notification Devices that are submitted to CBER (Hematology Division) Automated Apheresis (Blood Collection) Systems Disposables used in blood collection Laboratory Equipment Blood Establishment Computer software CDRH- 3700 510(k)s / year CBER- 100 510(k)s / year 6 Copyright © 2009 Haemonetics Corp. What else does CBER Review? BLA- Biologic License Applications Blood Centers submit for a license to manufacture blood products NDA’s- New Drug Applications Anticoagulant and Blood Nutrient solutions 510(k)s- Premarket Notifications Blood collection devices IDEs/INDs- Investigational Device Exemptions/ Investigation New Drug Devices and solutions PMAs – Premarket approval Not yet 7 Copyright © 2009 Haemonetics Corp. Substantially Equivalent??? “That’s a CDRH term… that doesn’t apply to CBER devices” 8 Copyright © 2009 Haemonetics Corp. Substantially Equivalent is part of the equation.. But more importantly… must meet Blood quality standards Hemolysis at the end of storage Residual White blood cell content Red cell recovery after filtration Total hemoglobin in the blood product platelet count 9 Copyright © 2009 Haemonetics Corp. Blood Quality standards can be found in… Guidance documents Memo’s to blood establishments Prior 510ks Transcripts from public meetings BUT not in the Code of Federal Regulations?? 10 Copyright © 2009 Haemonetics Corp. Scenario #1 Plasma- Secret criteria Automated device already cleared to collect plasma labeled as FFP (frozen within 6 hours) Very limited criteria published for plasma Clinical trial designed to qualify FFP and plasma frozen within 24 hours FDA has a host of parameters which now must be tested Communication with competitors reveals everyone has a slightly different list 11 Copyright © 2009 Haemonetics Corp. #2 In vivo recovery: Higher is better IDE submitted for a trial to qualify an apheresis device for collection of two units of red cells. Acceptance criteria includes in vivo recovery criteria which has been applied in submissions for 10+ years. Upon submitting 510(k) FDA informs us there is not more stringent criteria. …public session one slide contained a reference to more stringent criteria Communication with competitors reveals everyone has a slightly different criteria. 12 Copyright © 2009 Haemonetics Corp. #3 Tell me what you got and them we’ll tell you the criteria Public meeting a new criteria is revealed for platelets Pre-meeting with FDA is held concerning acceptance criteria for a clinical study Statistical boundaries were not defined FDA asked us to provide analysis with various confidence levels, 90%, 95% and 99% FDA determines criteria based on our analysis 13 Copyright © 2009 Haemonetics Corp. Effective Strategies Type C: pre- 510(k) meeting to discuss strategy Collaboration with competitors- let’s get in a room and hash this out. Offer to develop guidance documents through a working group Develop relationships with FDA to get a heads up about what initiatives are in process IDE submission Request for meta-analysis of data for products marketed Fight fire with fire: Statistician as a resource 14 Copyright © 2009 Haemonetics Corp. What can we learn from CBER Substantial equivalence is an antiquated term A new model will be developed to ensure safety and effectiveness for non-PMA devices Performance standards will be developed FDA may want to raise the bar… but this must be based on reality In God we trust all… all others bring data Access to a Statistician is critical FDA does not have enough resources.. get involved and help to develop standards. 15 Copyright © 2009 Haemonetics Corp. Thanks for your attention Questions?? Comments??? ECO FRIENDLY (Print): Click to add Conclusion Title Click to add Conclusion Second Level Third Level Fourth Level Fifth Level